Progressive leukoencephalopathy with intracranial calcification, congenital deafness, and developmental deterioration†
How to Cite this Article: Kuki I, Kawawaki H, Okazaki S, Kimura S, Nakano T, Fukushima H, Inoue T, Tomiwa K, Itoh M. 2011. Progressive leukoencephalopathy with intracranial calcification, congenital deafness, and developmental deterioration. Am J Med Genet Part A 155: 2832–2837.
Abstract
We report on a 12-year-old male with a unique cerebral white matter disease. His initial symptoms were congenital hearing loss and multiple intracranial calcifications on head CT. He developed severe intellectual disability and epilepsy. MRI showed signal abnormalities in the posterior limbs of the internal capsules, thalami, and cerebral white matter. The abnormalities were progressive over time. The neuropathology revealed diffuse and severe disruption of myelin and axons of the cerebral white matter and cerebrospinal tracts. We performed various metabolic examinations, detailed pathological investigations and genetic analyses, but could not identify the cause. To our knowledge his clinical course has not been described in the literature. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Leukodystrophy, a progressive disease involving white matter of the central nervous system, can be seen in metabolic, neurodegenerative, or genetic disorders [Kohlschütter et al., 2010]. Most leukodystrophies appear in childhood and show some progressive courses. Some recent experimental therapies such as enzyme replacement therapies and gene therapies, as well as the classical therapy of bone marrow transplantation, have been carried out, but with only limited efficacy. Magnetic resonance imaging (MRI) techniques have identified new brain diseases. Among them, several leukoencephalopathies have been identified by their characteristic white matter changes on MRI. These disorders are associated with deterioration of intellectual and motor abilities [Kohlschütter et al., 2010]. In particular, the pattern of MRI abnormalities is important for the identification and definition of the disease [Schiffmann and van der Knaap, 2009]. These novel MRI patterns, indicative of novel disorders, are associated with multiple novel genetic defects. Leukoencephalopathy with vanishing white matter (VWM) has characteristic features [Van der Knaap et al., 1997], and mutation of the subunits of eIF2B, the eukaryotic translational initiation factor, is a cause [Leegwater et al., 2001]. Also, Aicardi–Goutières syndrome (AGS) is a progressive leukoencephalopathy, which has intracranial calcification, lymphocytosis, and increased interferon (IFN)-α in the cerebrospinal fluid (CSF), and microangiopathy [Aicardi and Goutières, 1984; Goutières et al., 1998; Goutières, 2005; Lanzi et al., 2005; Rasmussen et al., 2005; Stephenson, 2008; Uggetti et al., 2009]. The causative genetic mutations of AGS are known to be TREX1 (AGS1), RNASEH2B (AGS2), RNASEH2C (AGS3), RNASEH2A (AGS4), and SAMHD (AGS5) [Rice et al., 2007, 2009].
We studied a male with a clinical course resembling AGS; however, the clinical features and laboratory data did not fulfill the criteria of AGS. The child showed congenital deafness, intellectual disability, and epilepsy accompanied with white matter degeneration and calcification, suggesting a unique leukoencephalopathy.
CLINICAL REPORT
A 12-year-old male was the second child from unrelated parents, and the older brother was healthy. The pregnancy was normal with no infectious episodes. When he was delivered at 41 gestational weeks, his birth weight was 3,115 g (near mean) and head circumference 34.2 cm (33.7 cm, mean for date). At 5 months, the infant had no head control and intracranial calcification was noticed on CT. He was recognized to have hearing loss. His developmental milestones were delayed; he achieved head control at 13 months of age; and walking without support at 30 months. He developed no meaningful speech. Around 3 years of age, he showed intractable epilepsy with spike waves on the occipital area. At 6 years, he developed spastic gait and showed spasticity of the lower limbs and ankle clonus. After an infectious episode, he demonstrated poor eye contact and persistent horizontal nystagmus.
The laboratory data revealed no significant abnormalities, and included lysosomal enzyme activities and very long chain fatty acid in serum, and N-acetyl-aspartate in urine. Levels of cytokines, interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-10, IL-12, IL-17a, IFN-α, IFN-γ, MCP-1, and TGF-β, in serum and CSF were normal. Neopterin and biopterin in CSF were also normal. Umbilical cytomegalovirus PCR analysis was negative. His electrophysiological data showed normal motor and sensory conduction velocities (MCV, SCV) of his median and sural nerves; 56 m/s (58.3 ± 5.4 of normal range) of median MCV, 46 m/s (49.5 ± 2.7 of normal range) of median SCV, 51 m/s (57.5 ± 6.9 of normal range) of sural MCV, and 38 m/s (42.4 ± 4.1 of normal range) of sural SCV. Head CT demonstrated calcification in the symmetrical periventricular regions surrounding low-density white matter and progressive atrophy (Fig. 1). The head SPECT showed low blood flow in the temporal and occipital lobes and thalami (Fig. 1). Head MRIs also exhibited progressive enlargement of the cerebral sulci and brainstem and extended abnormal signal in the white matter (Fig. 2). The signal abnormality and atrophy on MRI involved the occipital, frontal, parietal and temporal lobes, and posterior limbs of the internal capsules, including thalami. He was given anti-epileptic and anti-spastic agents, but his condition gradually declined. At 10 years, he showed status epilepticus and required respirator support. At 12 years, he suddenly died of tracheal bleeding.
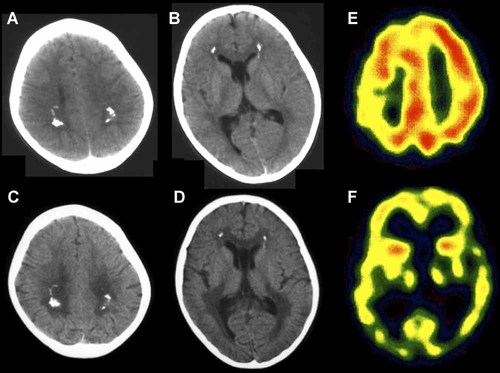
Brain imagings. At 5 years, the brain CT shows bilateral calcification in the periventricular white matter and mild ventricular dilatation (A,B). At 6 years, the calcification evidences no changes, but cerebral atrophy is progressive (C,D). SPECT exhibits marked decrease of intracranial blood flow on the temporal and occipital lobes, as well as thalami (6-year old; E,F).
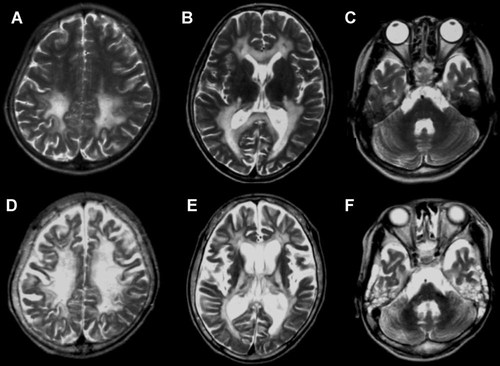
Serial brain MRIs. All figures are T2-weighted images. At 6 years, high intensity symmetrically appears in the white matter of the occipital and frontal lobes, as well as posterior limbs of the internal capsules, although subcortical white matter, cerebral cortex, cerebellum, and brainstem are spared (A–C). At 10 years, progressive atrophy and expanded pathological lesions are recognized in the white matter, cortex, and brainstem (D–F).
With the informed consent and permission of his parents, an autopsy was performed. A tracheo-brachiocephalic trunk fistula was revealed and suggested aortic bleeding as the cause of death. The other visceral organs, including heart, lung, liver, kidney, adrenal gland, bone marrow, spleen, and eye, revealed normal pathology without specific findings of congenital infection or metabolic disorders.
The neuropathological examination at autopsy revealed severe atrophy of the cerebrum and brainstem with enlargement of the ventricular system (brain weighing 1,025 g before fixation). We observed massive and severe disruption of myelin and axons with gliosis and lipid-laden macrophages in the white matter, excluding U-fibers, but not a spongy or edematous state (Fig. 3). The residual white matter showed marked gliosis with glial fibrillary acidic protein (GFAP)-immunohistochemistry (Fig. 3F). Some clusters of many minute mineralizations were found in the white matter, but were unrelated to vessels (Fig. 4). The internal capsules and corticospinal tracts were markedly involved. Thalami were also much involved, showing severe neuronal loss and gliosis. However, the cerebral cortices and basal ganglia were mild neuronal loss with gliosis (Fig. 4). In the brainstem, the corticospinal tracts demonstrated demyelination, but transverse fibers retained myelination. The substantia nigra showed depigmentation. In the cerebellum, the hemisphere showed mild loss of granule cells, but the cerebellar white matter was spared.
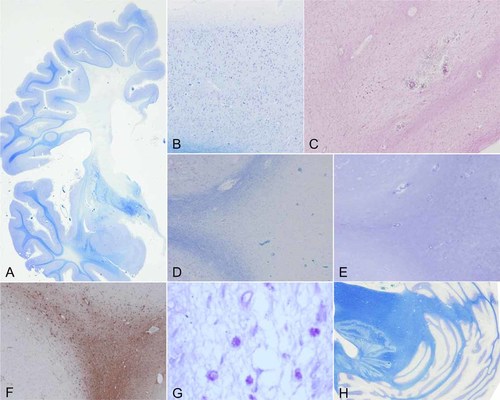
Histopathology of the cerebral white matter. The pathological degeneration is revealed as demyelination with gliosis in the deep white matter, but not in the subcortical white matter (A–C). The lesion shows marked gliosis (D–F) and appearance of many macrophages (G). The cerebellum is no significant change (H). A,B,D,H: Klüver–Barrera staining (KB), ×100 of original magnification; C: hematoxylin and eosin staining (HE), ×100; E: Holzer staining, ×100; F: GFAP-immunohistochemistry, ×100; G: PAS, ×400. All figures are of frontal lobe.
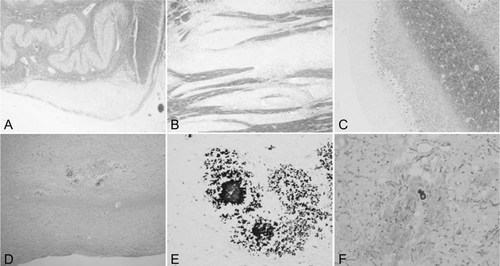
Histopathology of brainstem, cerebellum, and basal ganglia. The corticospinal tract, including the pyramis (A) and pons (B), shows marked demyelination, whereas the cerebellar white matter has normal myelination (C). Many calcifications are observed in the pons (D,E) and globus pallidus (F). A: KB, ×100; B: KB, ×200; C: KB, ×200; B: KB, ×200; D: HE, ×100; E: KB, ×200; F: KB, ×200.
We performed genetic analyses of TREX1 (AGS1), RNASEH2B (AGS2), RNASEH2C (AGS3), RNASEH2A (AGS4), and SAMHD (AGS5), as well as DARS2, after obtaining informed consent from his parents and the approval of the Ethical Committee of the Hospital and Institute. We did not find mutations in the genes examined.
We obtained permission to use all examination data from his parents and the ethical committee of our Hospital and Institute.
DISCUSSION
Our patient showed congenital hearing loss and severe developmental delay before abnormal signal appearance on brain imagings. His pathology showed involvement of the long-tract white matter, such as the corticospinal tract, whereas the short-tracts of U-fibers and transverse fibers in the pons were spared. The onset of his pathology is most likely of prenatal origin.
In light of his pathophysiology, it is important to define calcification in the symmetrical periventricular regions from initial imagings. Symmetrical calcifications in the cerebral white matter were not progressive. Many minute calcifications and these accumulations in his pathology might explain his calcification on CT. Some leukodystrophies described are reminiscent of our patient [Labrune et al., 1996; Leuzzi et al., 2000; Loes et al., 2003; Suzuki, 2003; Van der Knaap et al., 2003; Serkov et al., 2004; Adachi et al., 2006; Linnankivi et al., 2006; Scheper et al., 2007; Eichler et al., 2009; Van der Voorn et al., 2009]. AGS is relatively frequent and its causative genes have been identified [Rice et al., 2007, 2009]. AGS patients show lymphocytosis and a high level of IFN-α in CSF, as well as microangiopathy. AGS calcification is likely in the basal ganglia, and its pathology involves U-fiber from the early stage. From the genetic analyses of TREX1 (AGS1), RNASEH2B (AGS2), RNASEH2C (AGS3), RNASEH2A (AGS4), SAMHD (AGS5), our patient cannot be diagnosed as AGS, although some patients with definitive AGS have no mutations in the known genes.
On the other hand, leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation (LSBL) shows progressive cerebellar ataxia and involves the posterior column of the spinal cord; its causative gene is known as DARS2, an encoding gene of mitochondrial aspartyl-tRNA synthetase [Van der Knaap et al., 2003; Serkov et al., 2004; Scheper et al., 2007]. To our knowledge, LSBL has not been described with intracranial calcification and is different from our infratentorial pathology. In addition, our patient had no cerebellar pathology and no mutation of DARS2 gene. Leukodystrophy with ataxia and deafness (LAD) is also known to have congenital deafness, developmental delay, and epilepsy [Leuzzi et al., 2000]. However, the onset of LAD is in childhood, and its pathology involves diffuse white matter. Intrauterine infection leads to congenital deafness and brain malformation; but our patient showed no cytomegalovirus infection. Congenital infection does not have the progressive clinical course of our patient.
CONCLUSION
To our knowledge, the clinicopathological features of our patient have never been described to date in the literature. To our knowledge this report represents the first reported case of novel leukoencephalopathy with congenital deafness, developmental delay, and epilepsy with intracranial calcification.
Acknowledgements
We thank Dr. N. Sakai, Osaka University, for measurement of lysosomal enzyme activity, Dr. N. Shimozawa, Gifu University, for measurement of peroxysomal-related materials, and Dr. Y. Shigematsu, Fukui Medical University, for measurement of urine N-acetyl aspartate. This work was supported by a grant from Osaka City Medical Research.




