An 800 kb deletion at 17q23.2 including the MED13 (THRAP1) gene, revealed by aCGH in a patient with a SMC 17p†
How to Cite this Article: Boutry-Kryza N, Labalme A, Till M, Schluth-Bolard C, Langue J, Turleau C, Edery P, Sanlaville D. 2012. An 800 kb deletion at 17q23.2 including the MED13 (THRAP1) gene, revealed by aCGH in a patient with a SMC 17p. Am J Med Genet Part A 158A:400–405.
Abstract
We report on clinical and cytogenetic studies in a 7-year-old child with moderate intellectual disability, short stature, mild dysmorphism, and hearing loss. R-chromosome banding showed a de novo autosomal marker originating from the 17p chromosome segment in all cells analyzed. Array comparative genome hybridization (aCGH) was used to determine the gene content and proximal and distal breakpoints of the small supernumerary marker chromosome (SMC). These breakpoints mapped to the centromere of chromosome 17 and the 17p11.2 region, respectively. Unexpectedly, aCGH analysis also revealed a de novo deletion of 800 kb encompassing six genes in the 17q23.2 region, including MED13 (also known as THRAP1). We compared our patient with other reported cases of SMC(17), to determine the respective contributions of the duplication and the deletion to the phenotype. We cannot entirely exclude a minor role for the SMC(17), but we suggest that MED13 haploinsufficiency was responsible for the phenotype of the patient particularly the cataract, hearing loss and semicircular canal dysplasia. Moreover, this report highlights the usefulness of aCGH for the specification of gene content in cases of abnormality, facilitating the establishment of accurate phenotype–genotype correlations and the detection of other, complex rearrangements. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
The characterization of small supernumerary marker chromosomes (SMCs) discovered on karyotyping is a real challenge and is necessary for the establishment of genotype-phenotype correlations and for accurate genetic counseling. Indeed, only one third of SMCs are associated with a precise phenotype (e.g.: tetrasomy 12p and Pallister-Killian syndrome, chromosome 22 inversion-duplication, and cat-eye syndrome). This lack of phenotype–genotype correlation results from the extreme diversity of SMCs (degree of mosaicism, size, structure, origin, gene content) and the limitations of classical cytogenetic investigations [Crolla et al., 2005]. However, the development in recent years of cytogenetic tools, including array comparative genome hybridization (aCGH) in particular, has made it possible to characterize imbalanced chromosome abnormalities with a higher resolution (size, breakpoints), even in cases of mosaicism [Ballif et al., 2006; Menten et al., 2006]. Moreover, aCGH sometimes reveals other unexpected abnormalities associated with the markers [Baldwin et al., 2008; Tsuchiya et al., 2008] and the considerable phenotypic variability of patients with SMCs may be accounted for by these other “hidden” imbalanced rearrangements, which remained undetected before karyotyping. The case reported below illustrates this point.
We report here on clinical, cytogenetic and molecular studies in a girl with both a de novo SMC derived from the proximal short arm of chromosome 17 and a de novo 800 kb 17q23.2 deletion detected by aCGH. We also present additional evidence that the genes present on this SMC(17) probably have little, if any, phenotypic effect.
CLINICAL REPORT
This 7-year-old child was the second child of nonconsanguineous parents of French background with no informative family history. Her father was 34 and her mother was 31 years old at the time of conception. Intrauterine growth retardation was observed during pregnancy. The child was delivered normally at term. Her birth weight was 2,560 g (−2 SD), her birth length was 44.5 cm (−2 SD), and her head circumference was 32.2 cm (−2 SD). Blood lymphocyte karyotyping was performed at birth, due to possible sexual ambiguity (abnormality of the clitoris, subsequently considered to be an anatomic variant). A supernumerary marker chromosome was found in all the metaphase cells observed (40/40). Parental karyotypes were normal. Clinical follow-up showed significant feeding difficulties and recurrent bronchiolitis. Growth retardation (−2.5 SD), developmental delay (the patient sat unsupported at the age of 10 months walked at the age of 21 months), and delayed speech acquisition were noted at the age of three years. The patient also had a hearing loss, with prolonged latencies in auditory evoked potential tests. MRI scan showed bilateral semicircular canal dysplasia, with ossicular malformations. On clinical examination, we noted minor craniofacial abnormalities: temporal retraction, small ears with posterior rotation, unilateral preauricular fistula, small mouth, short nose, small hands and feet (no picture of the patient is provided because the required informed written consent could not be obtained).
On examination at the age of four years, the patient presented with pes equinus of the right foot, with loss of the Achilles and patellar tendon reflexes. Pes equinus was resolved by physiotherapy. A recent clinical examination of this child at the age of six years showed bilateral peripheral cataract and right conduction hearing loss (60–70 dB) requiring the use of a hearing aid. Speech and motor development improved with time. This patient had attention deficit and hyperactivity disorder (ADHD), which was improved by risperidone treatment. No sleep disorder was noted.
MATERIALS AND METHODS
Classical Cytogenetic Investigations
Peripheral blood lymphocytes were karyotyped by RHG banding, with standard techniques. Chromosome analyses were also performed on cultured blood lymphocytes from both parents.
Fluorescence In Situ Hybridization (FISH)
We initially carried out FISH with commercial probes, according to the manufacturer's instructions, to characterize the origin and gene content of the SMC. We used three probes: a chromosome 17 centromeric probe (D17Z1, KREATECH®), a Smith–Magenis locus-specific probe (FLI, SMR CYTOCELL®), and a Miller–Dieker locus-specific probe (LIS1, MDR CYTOCELL®)).
For confirmation of the aCGH data, we carried out FISH with the BAC clones RP11-458P8 (17p11.2), RP11-93H8 (17p11.2), and RP11-769P22 (17q21.31, control probe), as previously described [Romana et al., 1994]. We then used the RP11-342K2 BAC clone (17q23) encompassing the MED13 (THRAP1) locus, to check for MED13 deletion in the patient and for rearrangements of 17q23 in the samples from the parents.
Array Comparative Genomic Hybridization (aCGH)
Oligonucleotide aCGH was performed with the Agilent Human Genome CGH Microarray Kit 244K, according to the manufacturer's instructions (protocol version 4.0), as previously described [Schluth-Bolard et al., 2008]. Data were analyzed with the CGH Analytics software platform (Agilent®, Santa Clara, USA). Data were extracted with Feature Extraction® 9.1 software and analyzed with CGH analytics® 4.5 software, with the following parameters: window 0.2 MB, ADM2 threshold 6.0.
Quantitative PCR Analysis (qPCR)
qPCR was performed to confirm the DNA loss detected by aCGH analysis. We used probes binding to exon 8 of the MED13 gene, in accordance with the instructions provided with the kit (Quantitect SYBR GREEN PCR, Qiagen®, Courtabeouf, France). All qPCR were run on a LightCycler 2000 (Roche Applied Science®, Indianapolis, USA).
Microsatellite DNA Marker Analysis
We analyzed four microsatellites, to determine the parental origin of the abnormalities. We extracted genomic DNA from peripheral blood leukocytes from the patient and her parents. One of the microsatellites used (D17S1811; GenBank Accession No.: Z52802) mapped to the deletion and the other three microsatellites mapped to the duplicated region (D17S689: GenBank Accession No.: L29364; D17S1871: GenBank Accession No.: Z51496 and GATA70H05: GenBank Accession No.: G15778). Allele sizes were determined with GeneMapper® software.
RESULTS
The postnatal karyotype revealed the presence of two X chromosomes and a supernumerary small chromosome in all cells (40/40). This additional marker was too small for identification on the basis of its banding pattern (Fig. 1a). The karyotypes of the parents were normal. We therefore determined the origin of this de novo SMC by FISH with centromeric probes. The supernumerary chromosome hybridized with the chromosome 17 centromeric probe but not with the Smith–Magenis and Miller–Dieker microdeletion syndrome probes (Fig. 1c). We then carried out aCGH analysis, which revealed a gain of about 1.6 Mb of chromosomal material on 17p11.2, between the binding sites of oligonucleotides A_16_P40777061 and A_14_P113402 (respective positions 20,404,913 and 22,004,995 Mb; hg18, NCBI36; Fig. 1c), confirming partial trisomy 17p with the inclusion of euchromatic sequences. This result was confirmed by FISH (Fig. 1b). Interestingly, aCGH analysis also revealed the unexpected loss of 800 kb from the genomic sequence of 17q23.2, between the binding sites for oligonucleotides A_16_P03272064 and A_16_P20698245 (respective positions 57,372,913 and 58,206,595 Mb; hg18, NCBI36). This result was confirmed by qPCR with a primer binding to exon 8 of the MED13 gene and was shown to be a de novo modification (data not shown).
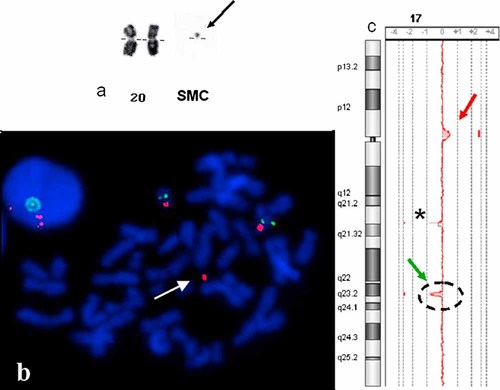
a: Partial karyotype (RHG banding) of the proband. The size of the SMC(17), indicated by an arrow, is compared with that of chromosome 20. b: FISH results for the RP11-93H8 (17p11.2, red) and RP11-769P22 (17q21.31, green) probes. The arrow indicates the SMC(17); c: aCGH analysis. The red arrow indicates the gain on 17p11.2. The green arrow indicates the loss on 17q23.2. * indicates a known CNP in a control DNA.
An analysis of parental samples with the BAC clone RP11-342K2 (17q23) revealed no major chromosome rearrangement, such as large inversions (data not shown). This clone was found to be absent from the patient's cells (data not shown).
The microsatellite analysis revealed that the deletion was of maternal origin. Unfortunately, the three microsatellites located in the same region of the genome as the SMC were not informative (Table I).
| Microsatellite marker | Location | Proband | Father | Mother | Parental origin |
|---|---|---|---|---|---|
| D17S1811 | 17q23.2 | 82 | 82/82 | 88/88 | Maternal |
| D17S689 | 17p11.2 | 169/169/169 | 169/171 | 169/169 | NI |
| D17S1871 | 17p11.2 | 353/353/353 | 353/371 | 353/353 | NI |
| GATA70H05 | 17p11.2 | 142/146/146 | 142/146 | 132/146 | NI |
- NI, not informative.
DISCUSSION
Eleven cases of chromosome 17-derived SMCs have been reported [Yatsenko et al., 2005; Kogan et al., 2009]. The 17p11.2 region is particularly rich in low-copy number repeats (LCRs). LCRs are DNA sequences involved in nonallelic homologous recombination (NAHR), a mechanism responsible for DNA rearrangements, such as deletions, duplications, inversions and SMCs. In the 17p11.2 region, the proximal and distal Smith–Magenis LCRs (SMS-REP) mediate the common deletion and reciprocal duplication resulting in the Smith–Magenis [Park et al., 2002] and Potocki–Lupski syndromes [Potocki et al., 2007], respectively. We describe here a case of SMC(17) with a distal breakpoint mapping to the centromere region and a proximal breakpoint mapping close to the proximal SMS-REP in the LCR17pD (Fig. 2). This SMC(17) therefore did not include the region generally implicated in Potocki–Lupski syndrome (PLS). To our knowledge, only two cases of SMC(17) that did not include the this region have been described to date [Shaw et al., 2004; Kogan et al., 2009].The case of Shaw et al. [2004] 2,170 had a distal breakpoint between the binding sites of clones CTD-2010G8 (19.9 Mb) and RP5-836L9 (20.1 Mb) and a centromeric proximal breakpoint. In our case, the distal breakpoint was located at 20.4 Mb, so the SMC had a lower euchromatin content (Fig. 2). In the other case, described by Kogan et al., there was a distal breakpoint in the proximal SMS-REP, as in our patient, but a proximal breakpoint in the 17q11.2 band. In addition to the mosaic SMC(17), the patient also displayed mosaicism for an SMC(13). The clinical features of these patients were not consistent with the phenotype of our patient. In particular, our patient presented with cataracts, severe hearing loss and semicircular canal dysplasia, which have never previously been reported in patients with SMC(17).
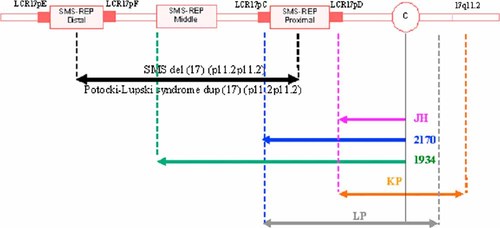
Schematic diagram of the 17p chromosomal region, derived from Yatsenko et al. [2005]. The black arrow shows the region most frequently involved in the Smith–Magenis and Potocki–Lupski syndromes. The purple arrows indicate the limits of the SMC(17) in the patient studied here (JH). The blue and green arrows indicate the limits of the SMC(17) reported by Shaw et al. [2004], the orange arrow indicates the limits of the SMC(17) corresponding to Kogan's patient [Kogan et al., 2009] (KP); the gray arrow indicates the boundaries of the SMC(17) reported in Thomas Liehr's chromosome marker website.
The duplicated region in our patient contained nine genes: MGC87631, USP22, DHRS7B, TMEM11, MGC33894, MAP2K3, KCNJ12, C17orf51, and FAM27L (RefSeqGenes, UCSC hg18). Six of these genes are encompassed by two copy number polymorphisms (CNPs) described by Redon et al. (CNPs 1218 and 1219). The other three genes (TMEM11, DHRS7B, and MGC33894) encode proteins with poorly characterized functions: TMEM11 encodes a protein thought to regulate the morphogenesis of mitochondria, particularly during cell stress (hypoxia, infection) [Rival et al., 2011]. DHRS7B (dehydrogenase/reductase member 7B) and MGC33894 encode proteins of unknown function (UCSC genome browser). In addition, a girl with a de novo min(17) encompassing the chromosomal region found in our patient was reported on Thomas Liehr's marker site (http://www.med.uni-jena.de/fish/sSMC/17.htm#Start17, case 17-O-p11.2/3-1). At the age of two years, the patient displayed no malformations, dysmorphism, or retardation of psychomotor development. We cannot entirely exclude the possibility that the SMC(17) plays a role in the intellectual disability of this patient, but we suggest that the phenotype observed in our patient, including the hearing loss and ocular abnormalities, was due to the de novo 800 kb 17q23.2 deletion rather than the SMC.
To our knowledge, no other small deletion has ever been described in this chromosomal region. This 17q23.2 deletion encompasses six genes (EFCAB3, MED13, METTL2A, TLK2, MRC2, and MARCH10, RefSeqGenes, UCSC hg18). A copy number polymorphism (CNP 1238) has been described in this region, but it is smaller than the observed deletion and includes only the EFCAB3 gene. The other five genes are absent from this CNP and are potential candidate genes underlying the clinical symptoms of this child. One of these genes, MED13 (OMIM 603808), is of particular interest with respect to the phenotype of the patient. Indeed, the product of this gene probably forms a subcomplex with the products of the MED12, CDK8, and Cyclin C genes—the human CDK8 subcomplex—which is thought to downregulate transcription [Knuesel et al., 2009]. These four genes appear to be essential for embryo development, but not for cell viability. In Drosophila, alteration to this complex cause malformations of the eyes and external sensory organs [Loncle et al., 2007]. Indeed, mutant Med13-Drosophilia display a lack of photoreceptor differentiation in the eye discs, leading to malformation of the ommatidia [Treisman, 2001]. These Med13-mutants also display downregulation of the bab gene in the leg discs. This gene is required for development of the proximo-distal leg axis and its downregulation results in distal leg shortening. A loss of bristle differentiation has also been observed in the adult notum: in Med13-mutants, expression of the sens gene, which is required for sensory organ precursor differentiation, is undetectable. Malformations of wings and antennas have also been noted. Interestingly, our patient presented with neurosensorial loss (hearing loss and cataracts). Moreover, one of the partners of MED13, MED12, interacts with Mediator, a protein involved in regulating neuronal gene expression [Ding et al., 2008]. MED12 mutations have been shown to be responsible for two X-linked intellectual disability disorders: the Opitz–Kaveggia and Lujan syndromes [Risheg et al., 2007; Schwartz et al., 2007].
Three other deletions of the MED13 gene were found in the DECIPHER database (Patients 249 237, 250 379, and 253 402). These three deletions were more proximal, but the only deleted region common to our patient contained only the MED13 locus. Two of the patients with these deletions presented mental retardation and deafness (Patients 249,237 and 250,379). No clinical data were available for case 253,402.
Our results provide no conclusive proof concerning the parental origin of the SMC. The chromosomal deletion was found to be of maternal origin, but our analyses of the parental samples found no chromosomal rearrangement involving the deleted region. We cannot exclude the possibility of a small parental inversion, but the presence of such an inversion could not account for the presence of both the SMC(17) and the 17q23 deletion. An in silico analysis of the deleted region with the TCAG website identified no highly similar duplicated sequences that might account for this deletion through an NAHR-based mechanism.
In summary, this patient's report highlights the complexity of phenotype-genotype correlations in microdeletion and microduplication syndromes. Our attention was initially drawn to the SMC(17), which was thought likely to be responsible for the patient's phenotype. However, our findings suggest that the basis of the abnormal phenotype in this patient, particularly as concerns the ocular and hearing abnormalities, is actually haploinsufficiency of the MED13 gene.
INTERNET ADDRESSES
-
UCSC: www.genome.ucsc.edu.
-
Thomas Liehr's marker site: www.med.uni-jena.de/fish/sSMC/17.htm#Start17.
-
TCAG site: http://projects.tcag.ca/cgi-bin/variation/gbrowse/hg18/.
-
DECIPHER site: http://decipher.sanger.ac.uk/.
Acknowledgements
We thank the members of this family for their continuing interest and cooperation. We thank the DGOS (Direction Générale de l'Organisation des Soins) for their support for the development of the aCGH platform at Lyon. We thank Prof. Zeynep Tümer (Center for Applied Human Molecular Genetics, Glostrup, Denmark) and Dr Anne Ronan (Hunter Genetic Unit from Waratah, NSW 2298, Australia), for accepting the citation of their DECIPHER Consortium cases (Numbers 250379 and 249237, respectively) in our discussion. Descriptions of these two cases will be provided elsewhere. We also thank the DECIPHER Consortium.




