Ring 21 chromosome presenting with epilepsy and intellectual disability: Clinical report and review of the literature†
How to Cite this Article: Specchio N, Carotenuto A, Trivisano M, Cappelletti S, Digilio C, Capolino R, Di Capua M, Fusco L, Vigevano F. 2011. Ring 21 chromosome presenting with epilepsy and intellectual disability: Clinical report and review of the literature. Am J Med Genet Part A 155:911–914.
To the Editor:
Several chromosomal abnormalities lead to anatomic alteration of the central nervous system, and most of them are accompanied by intellectual disability. Among patients with epilepsy and intellectual disability, about 6% have chromosomal abnormalities [Singh et al., 2002]. The majority of chromosome aberrations can be associated with a variety of epilepsy phenotypes and seizure types, but there are certain aberrations which show specific seizure and electroencephalographic (EEG) patterns. Chromosomal aberrations associated with epilepsy are mainly characterized by chromosome breaks and subsequent loss of genetic material. The more frequently reported deletions involve chromosomes 1 and 4 (del 1p36, del 4p16.3) [Battaglia and Guerrini, 2005]. Ring chromosomes could also be the cause of different epileptic phenotypes. Ring chromosomes 14, 17, and 20 are related to a well-defined epileptic electro-clinical picture [Singh et al., 2002]. Single cases have been published with ring chromosome 4 [Soysal et al., 2009], 6 [Kara et al., 2008], 9 [Lanzi et al., 1996], 18 [Nakayama et al., 1997], and 19 [Shahwan et al., 2004] associated with epilepsy.
Ring chromosome 21 [r(21)] was originally identified in children with dysmorphic features and congenital malformations. Subsequently it was also demonstrated in phenotypically normal individuals [Dallapiccola et al., 1986; Falik-Borenstein et al., 1992]. In a few patients it was also associated with epilepsy [Pardal Fernández et al., 2004]. We report on a patient with generalized epilepsy, intellectual disability, and dysmorphic features with 46,XY,r(21)(p13q22.3)/45,XY,−21 karyotype.
This report concerns a 13-year-old boy born at term after uneventful pregnancy and delivery, the third child of healthy nonconsanguineous parents. Family history was unremarkable. Birth weight was 3000 g, length 49 cm, and head circumference 34.5 cm. Motor skills were normal. Cognitive delay and language difficulties were noted since the age of 3 years. At 5 years of age, during sleep, he presented with a tonic–clonic generalized seizure lasting 3 min. In the following years, awake and asleep monthly tonic–clonic generalized seizures were reported. The patient was referred to our Hospital at the age of 11 years. Neurological examination was normal except for cognitive and speech impairment. His weight was 56.5 kg (>97th centile), height 154 cm (90th–97th centile), and head circumference was 54 cm (50th–75th centile). Clinical examination showed truncal obesity and facial anomalies (mild upslanting of the palpebral fissures, bifid nasal tip, hypoplastic philtrum) (Fig. 1).

Facial photograph of the patient showing mild upslanting of the palpebral fissures, bifid nasal tip, and hypoplastic philtrum.
Serial Video-EEGs revealed rare generalized spike-and-wave complexes lasting from 1 to 3 sec and apparently not associated with clinical signs (Fig. 2A). Intermittent photic stimulation evoked a photoparoxysmal epileptiform response (Fig. 2B–D). The patient was successfully treated with Valproate (700 mg/day). Brain MR was normal. Follow-up EEGs were normal. Genetic analysis for fragile X and Prader–Willi syndromes was negative. Karyotype analysis on lymphocytes revealed a mosaicism with 85% of the cell population with an r(21) chromosome. In particular, of 20 cells analyzed, 17 showed 46,XY,r(21)(p13q22.3), and 3 showed 45,XY,−21 karyotype. A deletion of the subtelomeric region in the long arm of the chromosome 21 was then confirmed with MLPA techniques.
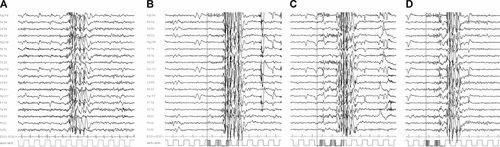
Interictal EEG recorded at the age of 11 years before starting antiepileptic medication. A: Generalized spike and wave complexes are evident lasting 3 sec and not associated with clinical manifestations. B–D: Photoparoxysmal epileptiform response evoked for IPS at 12–20 Hz frequency.
Leiter International Performance Scale-Revised (Leiter-R) test was performed at the age of 11 years and 9 months documenting intellectual disability (IQ = 38), confirmed by a Reasoning Fluid Score of 52. Currently he attends primary school with support. He has spatial and temporal orientation difficulties, as well as poor personal autonomies. He has had no further seizures during the past 2 years and is still on medication with Valproate.
Ring 21 chromosome, in both familial and isolated patients, results from a breakage and reunion of the short and long arms of the chromosome, leading to deletion of a variable amount of chromosome and haploinsufficiency [McGinniss et al., 1992]. A ring chromosome is unstable and generates different degrees of mosaicism that could explain the variability of the phenotype in r(21) patients. The size of deleted regions in patients with r(21) is the main characteristic of phenotypic variability. It has been proposed that hemizygosity for a critical region between q22.1 and q22.2 may be responsible for the “21q-syndrome”, which has a well-characterized phenotype [Theodoropoulos et al., 1995]. However, terminal deletions of short and long arms have also been reported in healthy people, suggesting that no important genes at the telomere of 21q have been lost. We reviewed all reports of patients with ring 21 in which symptoms involving the central nervous system were described. Overall 16 patients are reported. Table I summarizes the clinical findings of all the described patients. Epilepsy in the reported patients was characterized mainly by the recurrence of tonic and tonic–clonic seizures with focal signs [Aronson et al., 1987; Pardal Fernández et al., 2004], tonic–clonic generalized seizures [Schmid et al., 1983], myoclonic seizures [Kunze et al., 1975], and seizures facilitated by fever [Serra and Singh-Kahlon, 1976]. EEG revealed multifocal or generalized slow and epileptiform abnormalities in all patients; in one case photosensitivity was also reported [Kunze et al., 1975]. Two patients have been reported with EEG abnormalities characterized by paroxysmal and bisynchronous 2.5 Hz spike and wave discharges and diffuse delta waves [Palmer et al., 1977]. Epilepsy in our patient was characterized by the recurrence of tonic–clonic seizures and generalized EEG abnormalities with photoparoxysmal epileptiform response. Although this is a common EEG and clinical pattern for idiopathic generalized epilepsy, we suspect that it is related to the chromosomal aberration. The spectrum of epilepsy and EEG findings in the previously reported patients is wide: the recurrence of tonic–clonic generalized seizures and photosensitivity are the only common features with our patient.
| Author | Sex/age | Cytogenetic Finding | Neurological Findings | Associated Features |
|---|---|---|---|---|
|
Kunze et al., [1975 ] |
F/10 y | 46,XX,(r21) | Psychomotor retardation, myoclonic epilepsy, photosensitivity | Short stature, facial dysmorphic features, syndactyly, small narrow hands |
|
Serra and Singh-Kahlon [1976 ] |
F/11 y | 46,XX,r(21) | Severe psychomotor retardation, microcephaly, febrile seizures, epilepsy | Dysmorphic features, scoliosis, clinodactyly |
|
Palmer et al. [1977 ] |
F/20 d | 46,XX,r(21) | Minimal spasticity, abnormal EEG | Dysmorphic features, cardiopathy |
| F/5 y | 47,XXX,r(21) | Intellectual disability, uncoordinated gait, abnormal EEG | Dysmorphic features, syndactyly, thoracic spine fusion | |
| F/38 ya | 46,XX,r(21) | Mild intellectual disability | Dysmorphic features | |
|
Ferrante et al. [1983 ] |
M/21 y | 46,XY,r(21) | Mild psychomotor and intellectual disability, microcephaly | Dysmorphic features, clinodactyly, café-au-lait patches, |
|
Schmid et al. [1983 ] |
F/38 y | 46,XX,(r21)(p11q22.3) | Epilepsy, low normal intelligence | Short stature |
|
Aronson et al. [1987 ] |
M/3.5 m | 46,XY,(r21) | Holoprosencephaly, epilepsy, microcephaly | Dysmorphic features, scoliosis (hemivertebra T 10), diabetes insipidus, |
|
Dalgleish et al. [1988 ] |
M/3,5 y | 46,XY,r(21)(p1q22)/45,XY,−21 | Severe speech dyspraxia | Dysmorphic features, thrombocytopenia hypogammaglobulinemia |
|
Kennerknecht et al. [1990 ] |
M/6 m | 46,XY,r(21)(p13q22.3)mat | Psychomotor retardation, nystagmus | Minor dysmorphic features |
| F/n.a.a | 46,XX,r(21)(p13q22.3) | Normal | Normal | |
|
Falik-Borenstein et al. [1992 ] |
F/2 y | 46,XX,r(21)(p13q22)mat | Microcephaly | Severe growth retardation |
| F/n.a.a | 46,XX,r(21)(p13q22) | Microcephaly | Normal intelligence | |
|
Meire and Fryns, 1994 |
M/14 y | 46,XY,r(21)/45,XY,−21 | Axial hypertonia, psychomotor and intellectual disability, nystagmus, optic nerve hypoplasia | Dysmorphic features, severe growth retardation, lens dislocation |
|
Melkild [1994 ] |
F/4y | 46,XX/46,XX,−21,+r(21) | Serious speech and language problems | Dysmorphic features, bilateral hip-joint dysplasia |
|
Ohga et al. [1997 ] |
M/8y | 46,XY,r(21)(p11q22.2) | Psychomotor retardation, hypertonia | Hypogammaglobulinemia, thrombocytopenia, dysmorphic features |
|
Pardal Fernández et al. [2004 ] |
M/8y | 46,XY,r(21) | Progressive microcephaly, epilepsy | Dysmorphic features, short stature, vertebral and thorax malformation, cardiopathy, hypogammaglobulinemia |
- y, years; m, months; d, days; n.a., not available; F, female; M, male.
- a Mother of the propositus (upper line); EEG, Electroencephalogram; Th, Thorax.
The reported child represents a new case of epilepsy and intellectual disability associated with r(21). This finding strengthens the association of r(21) and epilepsy: few cases have been published until now, and seizure types and EEG findings are poorly described. A constant finding in patients with r(21) chromosome is loss of genetic material. These deleted regions could harbor candidate epilepsy genes, and therefore molecular studies should be performed in order to identify putative epilepsy genes.




