In this issue
MUTATION ANALYSIS MAY DISTINGUISH NF1 AND LEGIUS SYNDROME
Muram-Zbrovski et al (p. 1973) call for mutation analysis in some patients who have a clinical diagnosis of neurofibromatosis type 1 (NF1) but lack the tumorigenic and classic skeletal abnormalities of the disorder. Most patients with NF1 develop age-related neurofibromas and optic pathway tumors, although those with a specific 3-bp deletion in exon 22 of the NF1 gene at c.2970_2972delAAT have an attenuated phenotype with primarily pigmentary manifestations.
Due to the age-related occurrence of NF1 manifestations, young patients may also present with only pigmentary findings. It may be difficult to clinically distinguish presentations of NF1 without cutaneous neurofibromas from Legius syndrome. To identify patients who might have Legius syndrome and be less likely to develop tumors, the researchers sequenced NF1 exon 22 in an unselected cohort of 150 children and adolescents from a neurofibromatosis clinic who fulfilled clinical diagnostic criteria for NF1 but did not have SPRED1 mutations.
They identified 2 children with NF1 exon 22 mutations: an 11-year-old boy with the c.2970_2972 delAAT in-frame deletion and a 4-year-old boy with a c.2866dupA mutation in whole blood. The second boy's father had an attenuated form of NF1 and showed 24% germline mosaicism of the c.2866dup A mutation in whole blood. The attenuated phenotype and family history of these 3 individuals suggested that they could have had Legius syndrome. Recognizing the attenuated phenotype of NF1 mosaicism and confirmation by mutation analysis is necessary for medical management and family counseling.
CREATINE TRANSPORTER DEFICIENCY CASES DESCRIBED
Human fibroblasts have a single major creatine transporter, and measurement of its specific activity can confirm creatinine transporter deficiency, Ardon et al write (p. 1979).
In their report on 2 half-brothers with the condition, the authors note that 1 presented at 6 months of age with delays in development and has had slow progress since then with no regression. Seizures in 1 boy started at 3.5 years of age and responded well to treatment with anticonvulsants. He had failure to thrive, with all growth parameters—including head size—at or below the fifth centile. A brain MRI indicated hemispheric white matter abnormalities, while MR spectroscopy indicated markedly reduced creatine peak. Biochemical testing showed increased urine creatine/creatinine ratio, with normal plasma creatine and guanidinoacetate1.

Creatine transporter deficiency: family history and MR studies.
To confirm the diagnosis of creatine transporter deficiency, the authors measured [14]C-creatine transport in fibroblasts. [14]C-Creatine transport in normal human fibroblasts was linear for up to 2 hours at 37°C. Kinetic studies indicated the presence of a single saturable creatine transporter with a Km of 34.7 2.5 µM.
Fibroblasts from the first boy lacked creatine transport. DNA testing indicated hemizygosity for a novel deletion producing a frameshift (c.974_975delCA, p.Thr325SerfsX139) in the creatine transporter gene.
The patient's 12-year-old half-brother had similar biochemical and clinical abnormalities, except for the presence of macrocephaly and the absence of seizures. Their mother had normal development, but a history of seizures in childhood, the authors write.
FACIAL FEATURES ALONE DO NOT PREDICT NOONAN SYNDROME GENOTYPE
In patients with Noonan syndrome, facial phenotype alone is insufficient to predict the genotype, but certain facial features may facilitate an educated guess in some cases, write Allanson et al (p. 1960)2.
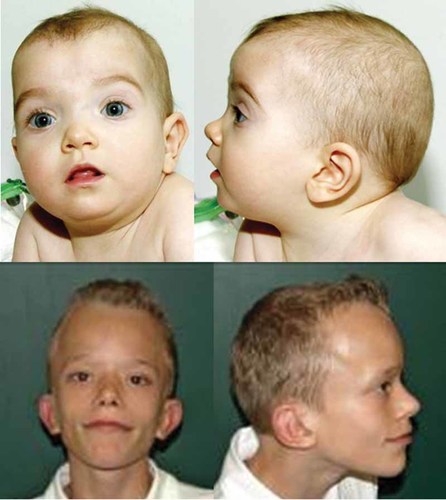
PTPN11 mutations and a facial gestalt uncharacteristic of Noonan syndrome.
Two research team members, dysmorphologists with experience with disorders of the Ras/MAPK pathway, evaluated photographs of 81 individuals with Noonan syndrome. The subjects in the photographs ranged from infancy to adulthood. Thirty-two members of the cohort have PTPN11 mutations, 21 have SOS1 mutations, 11 have RAF1 mutations, and 17 KRAS mutations.
The dysmorphologists judged the facial appearance of subject to be typical or atypical of Noonan syndrome. In each gene category, the researchers found both typical and unusual faces. Individuals with mutations in the most commonly affected gene, PTPN11—correlated with the cardinal physical features—may have a very atypical face. Conversely, some individuals with KRAS mutations—associated with a less characteristic intellectual phenotype and a resemblance to Costello and cardio-facio-cutaneous syndromes—can have a very typical face, the researchers found.




