Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations†
How to Cite this Article: Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK, Garg A. 2010. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet Part A 152A:2245–2253.
Abstract
Congenital generalized lipodystrophy (CGL) is a rare autosomal recessive disorder characterized by near total absence of body fat since birth with predisposition to insulin resistance, diabetes, hypertriglyceridemia, and hepatic steatosis. Three CGL loci, AGPAT2, BSCL2, and CAV1, have been identified previously. Recently, mutations in polymerase I and transcript release factor (PTRF) were reported in five Japanese patients presenting with myopathy and CGL (CGL4). We report novel PTRF mutations and detailed phenotypes of two male and three female patients with CGL4 belonging to two pedigrees of Mexican origin (CGL7100 and CGL178) and one pedigree of Turkish origin (CGL180). All patients had near total loss of body fat and congenital myopathy manifesting as weakness, percussion-induced muscle mounding, and high serum creatine kinase levels. Four of them had hypertriglyceridemia. Three of them had atlantoaxial instability. Two patients belonging to CGL178 pedigree required surgery for pyloric stenosis in the first month of life. None of them had prolonged QT interval on electrocardiography but both siblings belonging to CGL7100 had exercise-induced ventricular arrhythmias. Three of them had mild acanthosis nigricans but had normal glucose tolerance. Two of them had hepatic steatosis. All patients had novel null mutations in PTRF gene. In conclusion, mutations in PTRF result in a novel phenotype that includes generalized lipodystrophy with mild metabolic derangements, myopathy, cardiac arrhythmias, atlantoaxial instability, and pyloric stenosis. It is unclear how mutations in PTRF, which plays an essential role in formation of caveolae, affect a wide variety of tissues resulting in a variable phenotype. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Congenital generalized lipodystrophy (CGL) is a rare autosomal recessive disorder characterized by near total loss of body fat from birth. Mutations in three genes, 1-acylglycerol 3-phosphate-O-acyltransferase 2 (AGPAT2), Berardinelli–Seip congenital lipodystrophy 2 (BSCL2), and caveolin-1 (CAV1), were known at the time of our recent report of two Mexican-American siblings with a novel subtype of CGL associated with muscular weakness and cervical spine instability [Simha et al., 2008]. Since that publication, mutations in the polymerase I and transcript release factor (PTRF) gene were reported by Hayashi et al. 2009 resulting in a phenotype similar to our patients including generalized lipodystrophy and muscular dystrophy with elevated creatine kinase levels (CGL4). The report consists of five patients ranging in age from 8 years to 24 years old and three of them had muscle weakness with muscle mounding and muscle hypertrophy. None of the patients had mental retardation or acanthosis nigricans. Two of them had hepatosplenomegaly and one additional patient was reported to have fatty liver. Two of the older male patients had acromegaloid features with no androgynism. Two patients had arrhythmias and one of them developed atrial fibrillation. In addition, other variable manifestations such as constipation, nephrosis, umbilical prominence, scoliosis, recurrent pneumonia and transient immunoglobulin A deficiency were also reported [Hayashi et al., 2009].
In this report, we describe the phenotype of two new Mexican siblings and one Turkish female harboring novel homozygous null mutations in PTRF. In addition, we report novel compound heterozygous null mutations in PTRF in the two Mexican siblings previously described by us [Simha et al., 2008].
CLINICAL REPORTS
CGL178.5
This is an 11-year-old Hispanic boy, the son of nonconsanguineous Mexican parents (Figs. 1A,B and 2A,B). Reportedly, he was a normal looking baby at birth. He was born by cesarean at term gestation with a birth weight of 3 kg. The only complication during the terminal part of pregnancy was oligohydramnios. Two weeks after birth, he developed projectile vomiting and was diagnosed with pyloric stenosis. He then began losing sc fat from all over the body. He had surgery for pyloric stenosis at the age of 40 days. Following the surgery, he developed chronic diarrhea, especially after high fat meals, until the age of 3 years. He was noticed to be “stiff since birth” with reduced range of movement in all his joints. He was slower than other children of his age. He is currently in the 6th grade and doing well in school. He gets tired easily when riding a bicycle. He has had hypercholesterolemia since the age of 5 years for which he has not received any drug therapy thus far. He also has a right-sided hearing loss which is being evaluated and is currently not wearing a hearing aid.
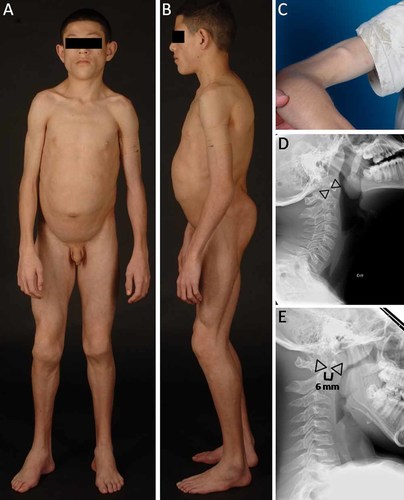
A: CGL178.5, anterior view of a 11-year-old boy showing generalized lipodystrophy, prominent muscularity, acromegalic features such as large hands and feet. B: CGL178.5, lateral view showing generalized lipodystrophy and protuberant abdomen. C: Percussion muscle mounding on the biceps of patient CGL178.5. D,E: Lateral radiographs of the cervical spine of CGL178.5 during extension and flexion of cervical spine, respectively, showing atlantoaxial instability. The interdental distance (shown by space between the triangles) was 4 mm during extension but was 6 mm during flexion. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
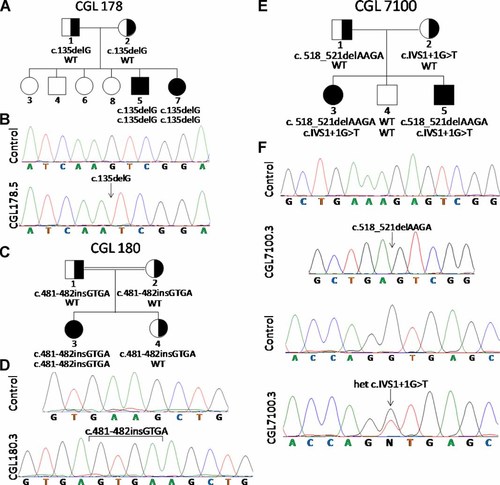
Pedigrees and sequence chromatograms of patients. A: 1: CGL178 pedigree. B: Normal chromatogram and that from CGL178.5 showing homozygous c.135delG PTRF mutation. C: CGL180 pedigree. D: Normal chromatogram and that from CGL180.3 showing homozygous insertion in exon 2 c.481-482insGTGA. E: CGL7100 pedigree. F: Chromatograms showing two normal sequences and those from CGL7100.3 showing heterozygous PTRF mutations c.518-521delAAGA (cloned chromatogram is shown) and c.IVS1 + 1G > T. WT, wild-type genotype. Circles denote females and squares denote males. Half filled indicates heterozygotes. Filled symbols indicate affected and unfilled symbols indicate unaffected subjects.
On examination he was 153.5 cm tall, weighed 41.9 kg, and had a head circumference in the 40th centile. His fundus examination was normal. Oral examination revealed enlarged tonsils bilaterally. Thyroid, lung, and cardiac examinations were normal with the exception of a short systolic murmur, grade 1/6. Liver was palpable 1 cm below the right costal margin and he did have umbilical prominence with a protuberant abdomen. His genitalia were prepubertal and testes were approximately 2 ml bilaterally. He had cavus-varus feet bilaterally and decreased range of motion at the ankles. Otherwise, joint examination was normal with no contractures. Painless muscle mounding was easily elicited by finger percussion or reflex hammer on several muscles over the thenar, forearm, arm, and thigh regions (Fig. 1C). He displayed good strength of 4/5 in the extremities. Muscle-stretch reflexes were normal. He was able to stand and ambulate independently with a “stiff” gait and calcaneo varus gait pattern. He had marked fat loss from his face, extremities, palms, and soles. He had an apparent muscularity and prominent veins consistent with generalized lipodystrophy. There was no acanthosis noted in the neck or groin; however, he had some minimal acanthosis in the axilla.
The boy underwent a standard 2-hr oral glucose tolerance test with 1.75 g/kg body weight glucose load, which was normal, but he had extreme fasting (74 µU/ml) and postprandial hyperinsulinemia (217–365 µU/ml). He had markedly low levels of high-density lipoprotein cholesterol (14 mg/dl) and had high serum triglycerides (1,074 mg/dl). Glycosylated hemoglobin A1c was normal (5.7%) but he had elevated creatine kinase (CK) level (625 U/L). Alanine aminotransferase level was 54 U/L and aspartate aminotransferase level was 35 U/L. Serum leptin level was 0.30 ng/mL and adiponectin level was 0.53 mg/L. His electrocardiograms did not show any signs of arrhythmia or evidence of QT prolongation (QTc = 433 msec). His liver fat on 1H magnetic resonance spectroscopy (MRS) was 33.8% and intramyocellular soleus fat was 0.82%. Dual energy X-ray absorptiometry (DEXA) study revealed total body fat of 12.7% (normal values, mean ± SD, being 30.0 ± 8.4, in age-matched Hispanic boys) [Kelly et al., 2009]. His cervical spine X-ray did show evidence of atlantoaxial instability with predental space measuring 4 mm in extension and increasing to 6 mm during flexion (Fig. 1D,E). He was however asymptomatic and had no neck pain or evidence of myelopathy.
Nerve conduction study of the upper and lower extremities was normal with the exception of a delayed distal latency of sensory action potentials in the ulnar and sural nerves. Needle electromyography revealed increased insertional activity and frequent rhythmic high-frequency (60–200 Hz) bursts of simple or complex motor unit potentials with variable amplitude lasting 60–750 msec consistent with neuromyotonia (Fig. 3A). They were mostly elicited by needle insertion.
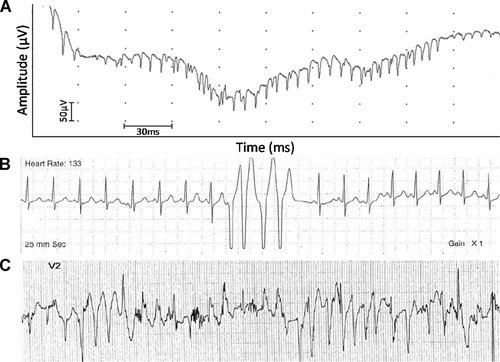
A: An electromyographic tracing from the biceps brachii showing neuromyotonic discharge. B: Holter monitor tracing of CGL7100.3 showing baseline normal rhythm with four consecutive beats of ventricular tachycardia. C: Electrocardiogram, lead V2 on treadmill stress test of CGL7100.3 after 3 min on Bruce Protocol at heart rate of 178 beats/min, 10 METs, speed of 3.4 m/hr, and 14% grade, showing bidirectional ventricular tachycardia suggestive of catecholaminergic polymorphic ventricular tachycardia (CPVT).
CGL178.7
This is a 16-month-old Hispanic girl, the younger sister of patient 178.5 (Fig. 2A). At birth, she appeared normal although mother's pregnancy was complicated by hypertension. She was born at 36 weeks of gestation with a birth weight of 3 kg. Her peripartum course was complicated by respiratory distress and she was admitted to the neonatal intensive care unit for a period of 3 days where she received antibiotic therapy. She subsequently developed vomiting and was diagnosed with pyloric stenosis and had surgery at 1 month of age. She had evidence of patent ductus arteriosus and patent foramen ovale on an echocardiogram done at birth to evaluate a heart murmur. Her developmental milestones were delayed. She started sitting at 12 months and started walking at 15 months. Her vocabulary consisted of about 5–7 words. Her appetite was not very good. Fat in her diet induced diarrhea. She now receives a low fat diet with high caloric supplements because of failure to thrive.
On examination she was 78.7 cm tall (25th centile) and weighed 8.3 kg (<5th centile). She had normal appearing eyes and low set ears. There was no acanthosis in the neck, axilla, or groin. Respiratory examination was normal. Cardiac examination revealed a 2/6 grade ejection systolic murmur. She had a protuberant abdomen with liver palpable 1 cm below the right costal margin. Genital examination revealed normal female anatomy with no clitoromegaly. Percussion muscle mounding was elicited in several upper and lower extremity muscles and she displayed no limitation in range of motion of her joints. Muscle-stretch reflexes were reduced throughout. She reached for objects with either hand and was able to ambulate independently with a wide based toddler gait. She had marked fat loss from the face and extremities; however, she did have some subcutaneous fat in her palms and soles.
She had low levels of HDL cholesterol (19 mg/mL), had slightly elevated serum triglycerides (142 mg/dl), and her serum insulin level was 8.9 µU/ml. Glycosylated hemoglobin A1c was normal (5.3%) but she had elevated CK level (571 U/L). Alanine aminotransferase level was 42 U/L and aspartate aminotransferase level was 37 U/L. Her serum leptin level was 0.44 ng/mL and serum adiponectin was 2.27 mg/L. Electrocardiogram showed no arrhythmias or prolongation of QT (QTc = 394 msec).
CGL180.3
This is an 11-year-old Turkish girl, the daughter of consanguineous parents (Fig. 2C,D). Reportedly, a generalized pattern of lipodystrophy was noted at birth. She was born full-term with a birth weight of 2,200 g. She had developmental delay and started walking at age 2. She has some difficulty walking due to scoliosis and has a tendency to walk on her toes. She has pes cavus.
On examination she was 138 cm tall and weighed 30.5 kg. She had percussion myoedema in her upper extremities, umbilical prominence and hepatomegaly. She had hyperextensibility of fingers and did not have acanthosis nigricans. Her fasting serum glucose was 88 mg/dl and she had a normal glucose tolerance test. Serum triglyceride level was 109 mg/dl. She had markedly elevated serum CK levels (2,337 U/L).
CGL7100.3
This pedigree and the affected patients were previously reported by us [Simha et al., 2008] (Fig. 2E,F). Currently, she is a 14-year-old Hispanic girl who has recently developed worsening hypertension requiring an increase in lisinopril dose from 2.5 mg twice daily to 5 mg twice daily. She recently achieved menarche and her periods are somewhat irregular.
On examination she was 160 cm tall and weighed 46.2 kg. We were able to elicit percussion-induced muscle mounding in the biceps. Since the last report, we determined her liver fat content using 1H MRS which was elevated at 16.64%. In addition, 1H MRS of soleus revealed muscle fat content of 1.04%. Her serum leptin level was 0.58 ng/mL and adiponectin level was 0.53 mg/L. On an electrocardiography, there was no evidence of QT prolongation (QTc = 429 msec). Her echocardiogram was normal for ventricular size and function. Holter monitoring for 24 hr revealed several nonsustained runs of a fast polymorphic ventricular tachycardia up to a heart rate of 300 beats/min (Fig. 3B). She had a prior Holter monitor at 3 years of age, which was essentially normal with the exception of rare premature ventricular contractions and sinus tachycardia. Therefore, an exercise study was conducted which revealed several runs of asymptomatic polymorphic ventricular tachycardia (Fig. 3C). This arrhythmia resembles catecholaminergic polymorphic ventricular tachycardia (CPVT). She has been started on nadolol to treat this condition.
CGL7100.5
This is an 8-year-old male was also previously reported by us [Simha et al., 2008] (Fig. 2E). He continues to have some learning disabilities. He has been unable to read at the level of his peers and has difficulty counting.
On examination he was 134.5 cm tall and weighed 28.2 kg. We were able to elicit percussion-induced muscle mounding in his biceps muscle. His liver fat on 1H MRS was 2.26% and intramyocellular soleus fat was 0.18%. His serum leptin level was 0.83 ng/mL and serum adiponectin level was 0.35 mg/L. On an electrocardiography, there was no evidence of QT prolongation (QTc = 403 msec). His echocardiogram was normal for left ventricular size and function. Holter monitoring for 24 hr revealed short runs of atrial tachycardia at rates of 180–220 beats/min. In addition, there appeared to be aberrant conduction of the atrial tachycardia versus occasional short runs of polymorphic ventricular tachycardia. Therefore, an exercise study was conducted which revealed asymptomatic short runs of wide complex tachycardia consistent with a polymorphic ventricular tachycardia. In addition, occasional atrial ectopy was noted. This arrhythmia also resembles CPVT and he has also been started on nadolol to treat this condition.
MATERIALS AND METHODS
All patients except CG180.3 were evaluated in the Clinical and Translational Research Center at the University of Texas Southwestern Medical Center at Dallas. Informed consent was obtained from the patients and parents. The study protocol was approved by the Institutional Review Board. Blood was collected after a 12-hr overnight fast for analysis of serum lipoproteins, insulin, glucose, a chemistry profile as previously described [Garg, 2000] and for genotyping.
Genomic DNA was isolated from blood using the Easy DNA kit from Invitrogen (Carlsbad, CA) according to the manufacturer's protocol. The coding region of the PTRF gene was amplified using gene-specific primers (available on request). The exon 1 was amplified in one fragment, whereas exon 2 was amplified in two overlapping fragments. The PCR conditions employed have been described before [Simha et al., 2003]. The purified PCR products were sequenced to determine the nucleotide alterations. The splice site prediction was made using web program NetGene (http://www.cbs.dtu.dk/services/NetGene2/).
Serum leptin and adiponectin were measured using radioimmunoassay kits (Millipore, Billerica, MA).
RESULTS
Sequencing of the exons and splice sites of PTRF gene showed a homozygous mutation in the CGL178 siblings: c.135delG, resulting in a short protein of p.Lys45AspfsX5 (Fig. 2B). CGL180.3 harbored a homozygous insertion in exon 2 c.481-482insGTGA which is predicted to result in a frame shift making an abnormal protein p.Lys161SerfsX41 (Fig. 2D). CGL7100 siblings showed compound heterozygous mutations, c.518-521delAAGA, which is predicted to result in frame shift making an abnormal protein of p.Lys173SerfsX101 and c.IVS1 + 1G > T, which is predicted to retain 143 nucleotides of intron 1, again resulting in the frame shift and an aberrant protein p.Asp158ValfsX49 (Fig. 2F).
The father of the proband from CGL178 pedigree (CGL178.1) is a 43-year-old male, who had a heterozygous mutation c.135delG (p.Lys45AspfsX5). He did not have lipodystrophy, but had poorly controlled diabetes with a hemoglobin A1c of 10%. His nonfasting serum cholesterol was 202 mg/dl, triglycerides were 405 mg/dl, and HDL cholesterol was 36 mg/dl. He had a normal CK level. The mother of the proband (CGL178.2) is a 40-year-old female with heterozygous mutation c.135delG (p.Lys45AspfsX5). She did not have lipodystrophy but did have slightly elevated nonfasting triglycerides of 206 mg/dl and a low HDL cholesterol level of 37 mg/dl. She did have a normal CK and glucose levels.
The parents and a sibling of the proband from CGL180 pedigree carried the heterozygous mutation c.481-482insGTGA. Phenotypic information on the parents and sibling was not available.
The 42-year-old father (CGL7100.1) of the probands belonging to CGL7100 siblings had the heterozygous mutation c.518-521delAAGA (p.Lys173SerfsX101). Clinically, he did not have lipodystrophy. He had slightly high triglyceride concentration of 215 mg/dl with normal HDL cholesterol, glucose, and CK levels. CGL7100.2 is a 35-year-old female and harbors the heterozygous mutation c.IVS1 + 1G > T (p.Asp158ValfsX49). She did not have lipodystrophy. She had slightly high serum triglyceride concentration of 200 mg/dl with normal HDL cholesterol, glucose, and CK levels.
DISCUSSION
Genetic, as well as phenotypic heterogeneity, is well described in patients with CGL. The two major subtypes, CGL1 and CGL2, harbor mutations in AGPAT2 and BSCL2, respectively, and have some overlapping and some distinct phenotypic features. For example, CGL2 patients lack any mechanical and metabolically active adipose tissue, whereas CGL1 patients lack only metabolically active adipose tissue and have well-preserved mechanical adipose tissue [Simha and Garg, 2003]. In addition, CGL2 patients have increased prevalence of cardiomyopathy and mental retardation, but they tend to have less pronounced lytic lesions in the appendicular skeleton [Van Maldergem et al., 2002; Agarwal et al., 2003]. Patients of either subtype, CGL1 or 2, develop marked metabolic complications such as severe insulin resistance, hypertriglyceridemia, acanthosis nigricans, premature diabetes, and hepatic steatosis. On the other hand, a single patient, of the subtype, CGL3, with a homozygous null mutation in CAV1 has been reported who had distinctive features compared to the two other major subtypes, such as, vitamin D resistance, hypocalcemia, reduced bone mineral density, preserved bone marrow fat, and short stature [Garg and Agarwal, 2008; Kim et al., 2008]. The recent report of a novel subtype of CGL by us [Simha et al., 2008] and the discovery of the fourth CGL locus, PTRF, by Hayashi et al. 2009 prompt the question about the phenotypic heterogeneity of CGL4 compared to the other CGL subtypes.
Only five patients were reported by Hayashi et al. 2009. Our report of an additional five patients adds other interesting features to what had been reported earlier. The main distinguishing feature of CGL4 is concomitant myopathy with weakness, inability to exercise, and percussion muscle mounding. Interestingly, all the patients with CGL4 reported so far have had null mutations in PTRF. This prompts the question whether less severe mutations such as missense mutations will be associated with CGL4 or with a less severe phenotype. Heterozygous carriers do not manifest overt lipodystrophy or myopathy; however, we cannot exclude mild metabolic disturbances like hypertriglyceridemia and diabetes amongst them. Similar data were reported previously [Hayashi et al., 2009]. Further studies comparing the phenotype of larger numbers of heterozygotes and normal subjects belonging to CGL4 pedigrees will determine the effect of haploinsufficiency of PTRF.
In contrast to our current patients, patients from Japan had no acanthosis and no metabolic abnormalities. Interestingly, four of our five patients have elevated triglycerides and two of the three patients evaluated for hepatic steatosis have elevated liver fat. Three of the five patients also have acanthosis nigricans in the setting of normal glucose tolerance. Two of them also had hyperinsulinemia and four of them had low leptin and adiponectin levels with normal adiponectin levels being about 7.8 mg/L (1.5–29.4) [Antuna-Puente et al., 2010]. Thus, although acanthosis nigricans appear to be mild compared to CGL1 and 2, our data suggest that these patients are also likely to develop severe hyperinsulinemia and metabolic abnormalities.
In addition, three of our five patients had atlantoaxial instability. CGL7100.3 was discovered to have atlantoaxial instability at a young age but was asymptomatic for some time. CGL7100.3 and CGL7100.5 were then involved in a car accident when they were 10 and 4 years old, respectively. CGL7100.3 had continued headaches and an MRI was done for evaluation. The atlantoaxial instability was seen again and due to perceived risk of quadriparesis in future, both patients were operated on to stabilize the cervical spine. Whether atlantoaxial instability poses a neurological risk and needs surgical correction remains unclear.
All of our five patients with PTRF mutations presented with CGL and myopathy (Table I). Two of the five patients had pyloric stenosis in the first month of life requiring corrective surgery and subsequently reported fat intolerance. Three of the five patients had normal echocardiograms with normal ventricular size and function. Only one of them had hypertension. None of them had QT prolongation, but on exercise testing two of them showed CPVT. CPVT is characterized by stress-induced ventricular tachycardia and has a mortality rate of 30% in untreated symptomatic patients [Swan et al., 1999; Kontula et al., 2005]. CPVT has been reported in patients with mutations in ryanodine receptor-2 (RYR2) gene [Priori et al., 2001] and rarely, in calsequestrin-2 (CASQ2) gene [Lahat et al., 2001]. Early initiation of oral β-adrenergic blockers is the treatment of choice [Napolitano and Priori, 2007]. Our data suggest that mutations in PTRF are also associated with CPVT.
| Characteristic | CGL178.5 | CGL178.7 | CGL180.3 | CGL7100.3 | CGL7100.5 |
|---|---|---|---|---|---|
| Age (years)/gender | 11/M | 1/F | 11/F | 14/F | 8/M |
| Pyloric stenosis | + | + | − | − | − |
| Atlantoaxial instability | + | n/a | n/a | + | + |
| Acanthosis nigricans | + | − | − | + | + |
| Hepatomegaly | + | + | + | + | + |
| Myopathy | + | + | + | + | + |
| Hypertension | − | n/a | − | + | − |
| Glucose tolerance | Normal | n/a | Normal | Normal | Normal |
| Hypertriglyceridemia | + | + | − | + | + |
| Echocardiogram | n/a | n/a | Normal | Normal | Normal |
| Catecholaminergic polymorphic ventricular tachycardia (CPVT) | n/a | n/a | n/a | + | + |
- M, male; F, female; +, present; −, absent; n/a, data not available.
Previously, Rajab et al. 2002 reported two interesting phenotypes of CGL patients from Oman. The patients reported as group A had many of the same characteristics that are well characterized for CGL1 or 2 such as muscle hypertrophy, acanthosis nigricans, raised insulin levels, diabetes mellitus, and hypertriglyceridemia [Rajab et al., 2002]. In contrast, 10 Group B CGL patients, age 0–14 years, had hypertrophic pyloric stenosis and also had cardiomegaly, reduced exercise tolerance, and elevated CK levels [Rajab et al., 2002]. Some older patients had arrhythmias and some of them died suddenly. Interestingly, none of them had acanthosis nigricans, diabetes mellitus, raised insulin levels, or hypertriglyceridemia. While our article was under review by the Journal, Rajab et al. 2010 reported mutations in PTRF in eight families with CGL and myopathy. However, detailed phenotypic characteristics were reported for only two patients. One 14-year-old boy from Oman had pyloric stenosis, prolonged QTc interval, and ventricular and supraventricular tachyarrhythmias. He had esophageal dysmotility and joint stiffness. He had insulin resistance as assessed by HOMA-IR but no acanthosis nigricans. He did have significant myopathy with elevated CK levels and hypertriglyceridemia. The second patient from the United Kingdom also had prolonged QTc with ventricular and supraventricular tachycardias and died at age 13 from sudden cardiac death due to ventricular fibrillation. She had atlantoaxial dislocation and hepatomegaly. She also had hypertriglyceridemia and insulin resistance, but no acanthosis nigricans. Four of the additional nine patients had elevated CK levels and joint contractures. Six of them had evidence of muscle mounding. Eight of them had hypertrophic pyloric stenosis, but none had atlantoaxial dislocation.
PTRF is an essential component of caveolae, specialized plasma membrane microdomains appearing as 50–100 nm vesicular invaginations, which function as endocytic carrier vesicles. Caveolae formation requires expression of caveolar proteins, caveolin (CAV) 1, 2, or 3 in various tissues. Mice lacking PTRF have profoundly reduced protein expression of all caveolins and no detectable caveolae [Liu et al., 2008]. It is likely that many manifestations in patients with CGL4 are due to disrupted caveolae formation and diminished expression of CAVs. Interestingly, mutations in CAV3 are associated with autosomal dominant myopathy [Carbone et al., 2000; Betz et al., 2001; Tateyama et al., 2002], hypertrophic cardiomyopathy [Hayashi et al., 2004], long QT syndrome [Vatta et al., 2006], and abnormal atrio-ventricular conduction [Catteruccia et al., 2009]. Mutations in CAV3 induce an increase in late Na current by affecting NAV1.5 channels present in caveolae and thus may delay cardiac depolarization and increase QT interval [Yarbrough et al., 2002; Lehnart et al., 2007]. It is possible that PTRF mutations induce arrhythmias by a similar mechanism or they may affect other cardiac ion channels that reside in caveolae including pacemaker channel (HCN4) [Barbuti et al., 2007], Ca2+ channel (CAv1.2) [Balijepalli et al., 2006], K+ channels (Kv1.5, Kir6.2/Sur2a) [Balijepalli and Kamp, 2008], and Na+/Ca2+ exchanger (NCX) [Bossuyt et al., 2002; Camors et al., 2006].
In addition, PTRF is expressed mostly in adipocytes and muscle tissue including smooth muscle. There has been evidence of patients showing smooth muscle hypertrophy in the gastrointestinal tract leading to dysmotility, dysphagia, and ileus [Rajab et al., 2002]. It is unclear to us whether the fat intolerance reported by two of our patients (CGL178) is due to smooth muscle dysfunction.
In summary, we report five additional CGL patients with novel PTRF mutations who shared some of the same clinical characteristics as previously reported in five patients from Japan such as CGL, myopathy, percussion muscle mounding, ankle contractures, and acromegaloid features. We now add additional clinical features associated with CGL4, such as acanthosis nigricans, hypertriglyceridemia, hyperinsulinemia, hepatic steatosis, hypertension, atlantoaxial instability, pyloric stenosis, neuromyotonia, and CPVT. The heterogeneity even within our own patients illustrates the phenotypic variability within this rare clinical syndrome. The molecular mechanisms by which PTRF mutations cause manifestations in various organ systems and phenotypic variability remain to be elucidated.
Acknowledgements
We thank Dr. Gregory L. Johnson for help in the cardiac evaluation of the affected patients belonging to pedigree CG7100. We thank Lidia Szczepaniak, Ph.D. for interpretation of the liver and muscle MRS studies and Claudia Quittner for her assistance during patient evaluations. We thank Dr. Gulcin Akinci for evaluation of patient belonging to CGL180 pedigree. We also thank Sarah Masood and Crystal Kittisopikul for help with illustrations and mutational screening. This work was supported by the National Institutes of Health grants R01-DK54387, CTSA Grant UL1 RR024982, and Southwest Medical Foundation.




