Mechanisms underlying early development of pulmonary vascular obstructive disease in Down syndrome: An imbalance in biosynthesis of thromboxane A2 and prostacyclin†
How to Cite this Article: Fukushima H, Kosaki K, Sato R, Yagihashi T, Gatayama R, Kodo K, Hayashi T, Nakazawa M, Tsuchihashi T, Maeda J, Kojima Y, Yamagishi H, Takahashi T. 2010. Mechanisms underlying early development of pulmonary vascular obstructive disease in Down syndrome: An imbalance in biosynthesis of thromboxane A2 and prostacyclin. Am J Med Genet Part A 152A:1919–1924.
Abstract
Patients with Down syndrome (DS) and a left-to-right shunt often develop early severe pulmonary hypertension (PH) and pulmonary vascular obstructive disease (PVOD); the pathophysiological mechanisms underlying the development of these complications are yet to be determined. To investigate the mechanisms, we evaluated the biosynthesis of thromboxane (TX) A2 and prostacyclin (PGI2) in four groups of infants, cross-classified as shown below, by measuring the urinary excretion levels of 11-dehydro-TXB2 and 2,3-dinor-6-keto-PGF1α: DS infants with a left-to-right shunt and PH (D-PH, n = 18), DS infants without congenital heart defect (D-C, n = 8), non-DS infants with a left-to-right shunt and PH (ND-PH, n = 12), and non-DS infants without congenital heart defect (ND-C, n = 22). The urinary excretion ratios of 11-dehydro-TXB2 to 2,3-dinor-6-keto-PGF1α in the D-PH, D-C, ND-PH, and ND-C groups were 7.69, 4.71, 2.10, and 2.27, respectively. The ratio of 11-dehydro-TXB2 to 2,3-dinor-6-keto-PGF1α was higher in the presence of DS (P < 0.001), independently of the presence of PH (P = 0.297). The predominant biosynthesis of TXA2 over PGI2, leading to vasoconstriction, was observed in DS infants, irrespective of the presence/absence of PH. This imbalance in the biosynthesis of vasoactive eicosanoids may account for the rapid progression of PVOD in DS infants with a left-to-right shunt. © 2010 Wiley-Liss, Inc.
INTRODUCTION
About one-half of Down syndrome (DS) patients have congenital heart defects, most of which are associated with a left-to-right shunt such as a complete atrioventricular septal defect, a ventricular septal defect, a patent ductus arteriosus, or an atrial septal defect. Whereas a large left-to-right shunt leads to increased pulmonary blood flow and, hence, pulmonary hypertension (PH) and eventually pulmonary vascular obstructive disease (PVOD), it has long been suggested that DS patients with a left-to-right shunt develop PVOD earlier in their clinical course than non-DS patients with a left-to-right shunt [Chi and Krovetz, 1975; Clapp et al., 1990; Suzuki et al., 2000]. Of particular importance, infants with DS have been suggested to be at risk of developing persistent pulmonary hypertension of the newborn even in the absence of congenital heart defects [Shah et al., 2004], implying a functional abnormality of the pulmonary vessels that is specific to DS.
Two vasoactive eicosanoids, thromboxane A2 (TXA2) and prostacyclin (PGI2), have been postulated to play important roles in maintaining pulmonary vascular function. TXA2 is synthesized mainly by activated platelets and acts as a vasoconstrictor, a promoter of platelet aggregation [Hamberg et al., 1975], and a stimulator of vascular smooth muscle cell proliferation [Sachinidis et al., 1995; Pakala et al., 1997]. PGI2 is mainly synthesized by endothelial cells, including pulmonary arterial endothelial cells, and acts as a vasodilator, an inhibitor of platelet aggregation [Moncada et al., 1976], and an inhibitor of vascular smooth muscle cell proliferation [Sinzinger et al., 1987; Wharton et al., 2000]. Thus, TXA2 and PGI2 have opposing effects and the balance of their biosynthesis plays an important role in the modulation of vascular tone and platelet aggregation. Actually, the TXA2:PGI2 biosynthesis ratio is reportedly high in patients with primary (idiopathic) and secondary pulmonary hypertension, for example, collagen vascular diseases [Christman et al., 1992]. A few reports have investigated the biosynthesis of vasoactive eicosanoids among children with congenital heart disease, and an elevated TXA2 level has been reported in such children [Adatia et al., 1993]. However, the TXA2:PGI2 biosynthesis ratio has never been investigated in children with DS.
TXA2 and PGI2 have very short half-lives in the blood circulation, and the biosynthesis of both TXA2 and PGI2 exhibits circadian variations. Therefore, only the stable breakdown products or metabolites of these eicosanoids, and not the eicosanoids themselves, can be measured accurately. Furthermore, stimulation of the endothelium and platelets during blood sampling can induce artifactual increases in the concentrations of TXA2 and PGI2. For these reasons, we measured the urinary excretion of 11-dehydro-TXB2 and 2,3-dinor-6-keto-prostaglandin (PG) F1α in 24-hr urine samples as indices of extrarenal TXA2 and PGI2 biosynthesis, respectively. The purpose of the present study was to investigate the possible existence of an imbalance in the biosynthesis of TXA2 and PGI2 in DS patients with congenital heart defect and a left-to-right shunt and to evaluate whether the biosynthesis of TXA2 and PGI2 may differ in DS patients and non-DS patients by measuring the urinary excretion of 11-dehydro-TXB2 and 2,3-dinor-6-keto-PGF1α in 24-hr urine samples.
MATERIALS AND METHODS
Patients
We used a cross-sectional survey design for this study. The subjects consisted of four groups of patients as follows: consecutive DS infants with a left-to-right shunt and PH (D-PH group, n = 18; 11 boys, 7 girls; 2–24 months, median of 6 months), DS infants without congenital heart defect (control) (D-C group, n = 8; 3 boys, 5 girls; 6–20 months, median of 11 months), consecutive non-DS infants with a left-to-right shunt and PH (ND-PH group, n = 12; 6 boys, 6 girls; 2–14 months, median of 6.5 months), and non-DS infants without congenital heart defect (control) (ND-C group, n = 22; 18 boys, 4 girls; 3–32 months, median of 10 months). None of the infants had renal insufficiency or had taken nonsteroidal antiinflammatory drugs, including aspirin, for at least 2 weeks before the study. The study was conducted in accordance with the Declaration of Helsinki and its amendments. Informed consent for participation in the study was obtained from the legal guardian(s) of each infant.
In most patients, the cardiac defects were diagnosed using cardiac catheterization, in addition to echocardiography. PH was diagnosed when the mean pulmonary artery pressure was greater than 25 mmHg at cardiac catheterization, or an estimated right ventricular systolic pressure was greater than 40 mmHg and/or interventricular septal flattening was present in echocardiography. All infants with cardiac defects underwent successful surgical repairs after the collection of the urine specimens. No patients with Eisenmenger syndrome or serious non-cardiac disorders were enrolled in the study.
The diagnosis of DS was confirmed by cytogenetic analysis. The absence of DS was determined based on the results of a thorough physical examination by a clinical geneticist. Because of ethical concerns, no cytogenetic analyses were performed in these infants. The absence of congenital heart defects and PH was confirmed by echocardiography in DS infants. The non-DS control infants were selected based on the infant's negative medical history, normal results of electrocardiography evaluation and thorough physical examination by a pediatric cardiologist who specializes in pulmonary hypertension. A 5-year follow-up of these non-DS control subjects revealed the healthy status of the control subjects.
Analysis of Eicosanoid Metabolites
In each case, a well-mixed sample of 20–30 ml of urine was stored at −20°C immediately after 24-hr urine collection. The urinary concentrations of 11-dehydro-TXB2 and 2,3-dinor-6-keto-PGF1α were measured using a radioimmunoassay (11-dehydro-TXB2 [125I] RIA Kit, Perkin Elmer; 6-Keto-PGF1α [125I] Biotrak Assay System with Magnetic Separation, Amersham Biosciences). A radioimmunoassay combined with proceeding sample purification using a specific monoclonal antibody has been previously reported to provide reliable measurements of these eicosanoids levels comparable to those obtained using gas chromatography–mass spectrometry [Hiramatsu et al., 1994; Perneby et al., 1999]. The urinary creatinine concentrations were measured using routine clinical chemistry techniques. The excretion levels of 11-dehydro-TXB2 and 2,3-dinor-6-keto-PGF1α were expressed as nanograms per gram creatinine to allow for changes in renal function and urine volume. All the measurements in the patients with heart defects were made preoperatively.
Statistical Analysis
The results are shown as the mean ± SD. The patients were cross-classified, as previously described, into D-PH, D-C, ND-PH and ND-C groups. The urinary excretion levels of 11-dehydro-TXB2, 2,3-dinor-6-keto-PGF1α, and their ratio were analyzed using a two-way analysis of variance (ANOVA) [Sheskin, 2004] with the factors “DS” (Down syndrome vs. controls) and “PH” (pulmonary hypertension vs. controls). To control for the possible additive effect of having both “DS” and “PH” factors, interaction terms were added to the model. Natural logarithmic transformations of the excretion levels of 11-dehydro-TXB2, 2,3-dinor-6-keto-PGF1α and their ratio were used to improve normality before performing the statistical analysis. A subgroup analysis for DS was performed using the Student's t-test as a post-hoc comparison. The statistical analyses were performed using SAS 8.0 software. Differences were considered significant at P < 0.05.
RESULTS
The clinical findings and eicosanoid excretion levels in the D-PH group and the ND-PH group are shown in Table I. The background characteristics (age and sex) of both groups were comparable. The pulmonary arteriolar resistance was higher in the D-PH group than in the ND-PH group (4.5 ± 1.6 vs. 3.5 ± 1.6 Wood units m2, P = 0.04), whereas the mean pulmonary artery pressures were not significantly different (53.6 ± 12.5 in the D-PH vs. 44.3 ± 8.5 mmHg in the ND-PH group, P = 0.07).
| Age, mo | Sex | Diagnosis | Systemic arterial pressure, S/D (m), mmHg | Pulmonary arterial pressure, S/D (m), mmHg | Aortic oxygen saturation (%) | Qp/Qs | Pulmonary vascular resistance, wood (units m2) | 11-Dehydro- TXB2 (ng/g Cr) | 2,3-Dinor-6-keto-PGF1α (ng/g Cr) | Ratio of TXB2 to PGF1α |
|---|---|---|---|---|---|---|---|---|---|---|
| Down syndrome infants with PH (D-PH group) | ||||||||||
| 2 | M | AVSD | 84/— | NA | 95 | NA | NA | 5,530 | 553 | 10.00 |
| 3 | M | AVSD | 84/43 | 73/28 (47) | 96 | 4.1 | 2.4 | 10,674 | 1,236 | 8.64 |
| 4 | F | VSD | 80/— | NA | 99 | NA | NA | 5,699 | 1,140 | 5.00 |
| 5 | F | VSD, PDA | 98/40 (63) | 86/35 (58) | 96 | 1.8 | 4.5 | 10,933 | 933 | 11.71 |
| 5 | M | VSD, ASD (os) | 96/43 | 73/33 (51) | 92 | 1.6 | 9.0 | 9,881 | 328 | 30.12 |
| 5 | M | VSD | 97/64 | 81/46 (65) | 96 | 1.6 | 5.6 | 6,584 | 988 | 6.67 |
| 5 | F | VSD, PDA, ASD (os) | 83/34 (56) | 82/35 (55) | 87 | 2.2 | 3.9 | 3,190 | 601 | 5.31 |
| 5 | M | VSD | 101/59 | 55/20 (36) | 94 | 2.4 | 3.4 | 3,003 | 767 | 3.92 |
| 6 | F | AVSD | 105/68 | 102/60 (78) | 93 | 1.6 | 6.5 | 5,191 | 527 | 9.86 |
| 6 | F | VSD, PDA | 83/42 (58) | 85/50 (65) | 100 | 2.3 | 4.0 | 4,124 | 2,474 | 1.67 |
| 7 | M | VSD, PDA | 59/34 (45) | 60/23 (42) | 97 | 3.0 | 3.9 | 3,900 | 800 | 4.88 |
| 8 | M | VSD | 70/45 | 69/23 (43) | 93 | 3.3 | 5.7 | 16,216 | 2,811 | 5.77 |
| 11 | F | PDA | 115/50 (78) | 97/48 (68) | 100 | 2.6 | 5.1 | 11,327 | 1,230 | 9.21 |
| 12 | M | VSD | 90/50 | 71/33 (52) | 96 | 2.7 | 4.8 | 5,138 | 1,581 | 3.25 |
| 12 | M | PDA | 92/54 (70) | 82/48 (62) | 93 | 3.2 | 3.5 | 6,711 | 984 | 6.67 |
| 16 | F | ASD (op) | 86/55 | 51/17 (30) | 95 | 2.9 | 2.3 | 16,732 | 3,113 | 5.38 |
| 17 | M | ASD (op) | 93/52 | 80/28 (51) | 96 | 2.0 | 3.7 | 5,183 | 785 | 6.60 |
| 24 | M | VSD | 85/38 | 75/49 (55) | 94 | 1.8 | 4.4 | 13,714 | 3,829 | 3.58 |
| Mean | 7,985 | 1,371 | 7.69 | |||||||
| SD | 4,378 | 1,003 | 6.19 | |||||||
| Non-Down syndrome infants with PH (ND-PH group) | ||||||||||
| 2 | M | VSD, ASD(os) | 60/35 | NA | 100 | NA | NA | 6,009 | 1,717 | 3.50 |
| 2 | F | VSD, PDA | 100/— | NA | 98 | NA | NA | 6,429 | 1,643 | 3.91 |
| 3 | M | VSD | 88/— | NA | 100 | NA | NA | 6,278 | 2,691 | 2.33 |
| 4 | M | VSD | 88/— | NA | 92 | NA | NA | 9,125 | 3,612 | 2.53 |
| 5 | M | VSD | 104/63 | 60/30 (43) | 97 | 2.0 | 1.5 | 2,308 | 1,282 | 1.80 |
| 6 | F | AVSD | 91/61 | 65/33 (47) | 96 | 2.7 | 6.1 | 2,810 | 1,601 | 1.76 |
| 7 | F | AVSD | 97/59 | 83/45 (57) | 95 | 3.1 | 4.2 | 5,161 | 3,871 | 1.33 |
| 9 | M | VSD | 97/50 | 52/24 (38) | 95 | 1.9 | 3.0 | 3,202 | 3,202 | 1.00 |
| 9 | M | VSD | 85/51 | 53/18 (34) | 97 | 4.4 | 1.2 | 1,343 | 1,194 | 1.12 |
| 9 | F | VSD | 80/50 | 70/26 (48) | 96 | 3.2 | 3.1 | 9,043 | 3,723 | 2.43 |
| 11 | F | VSD | 87/56 | 77/29 (53) | 100 | 2.9 | 2.7 | 7,273 | 3,068 | 2.37 |
| 14 | F | VSD | 97/62 | 54/24 (34) | 100 | 2.3 | 2.4 | 2,230 | 1,990 | 1.12 |
| Mean | 5,101 | 2,466 | 2.10 | |||||||
| SD | 2,687 | 1,003 | 0.93 | |||||||
- S, systolic; D, diastolic; m, mean; Qp, pulmonary blood flow; Qs, systemic blood flow; AVSD, complete atrioventricular septal defect; VSD, ventricular septal defect; PDA, patent ductus arteriosus; ASD (os), atrial septal defect, ostium secundum type; ASD (op), atrial septal defect, ostium primum type; NA, no cardiac catheterization (PH was diagnosed by echocardiography).
The mean urinary 11-dehydro-TXB2 excretion levels in the D-PH, D-C, ND-PH, and ND-C groups were 7,985 ± 4,378, 3,504 ± 1,400, 5,101 ± 2,687, and 1,663 ± 619 ng/g of creatinine, respectively (Fig. 1a). The urinary 11-dehydro-TXB2 excretion level was higher both in the presence of DS (P < 0.0001) and in the presence of PH (P < 0.0001). No significant interaction between the presence of DS and the presence of PH was observed (P = 0.326), thus the presence of DS and PH affected urinary 11-dehydro-TXB2 excretion levels in independent manners.
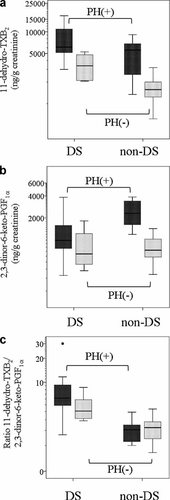
Figures showing boxplots of (a) urinary excretion levels of 11-dehydro-TXB2, (b) urinary excretion levels of 2,3-dinor-6-keto-PGF1α, and (c) ratios of 11-dehydro-TXB2 to 2,3-dinor-6-keto-PGF1α excretion in each of the four groups (from left to right: DS infants with a left-to-right shunt and PH [D-PH group], DS infants without congenital heart disease [D-C group], non-DS infants with a left-to-right shunt and PH [ND-PH group] and non-DS infants without congenital heart disease [ND-C group]). The boxes indicate the interquartile ranges, the horizontal line inside each box indicates the median, the I bars indicate the largest/smallest values in the 1.5-times interquartile from the edge of the box, and the additional points indicate extreme values. TX, thromboxane; PG, prostaglandin; DS, Down syndrome; PH, pulmonary hypertension.
The mean urinary 2,3-dinor-6-keto-PGF1α excretion levels in the D-PH, D-C, ND-PH, and ND-C groups were 1,371 ± 1,003, 876 ± 571, 2,466 ± 1,003, and 810 ± 294 ng/g of creatinine, respectively (Fig. 1b). A significant interaction between the presence of DS and the presence of PH (P = 0.018) was observed, and the effect of PH on the urinary 2,3-dinor-6-keto- PGF1α excretion levels was less pronounced in the presence of DS. The urinary 2,3-dinor-6-keto-PGF1α excretion level was lower in the presence of DS (P = 0.012) and higher in the presence of PH (P < 0.0001).
The urinary 11-dehydro-TXB2:2,3-dinor-6-keto-PGF1α excretion ratios in the D-PH, D-C, ND-PH, and ND-C groups were 7.69 ± 6.19, 4.71 ± 2.00, 2.10 ± 0.93, and 2.27 ± 1.00, respectively (Fig. 1c). The ratio of 11-dehydro-TXB2 to 2,3-dinor-6-keto-PGF1α was higher in the presence of DS (P < 0.001) and independent of the presence of PH (P = 0.297). The P-value of the interaction analysis between the presence of DS and the presence of PH was 0.141, implying that an interaction was present. In a subgroup analysis among infants with DS, patients with PH tended to have a higher ratio than infants without congenital heart disease, even though statistical significance was not achieved (P = 0.086).
DISCUSSION
The present study demonstrated that the urinary 11-dehydro-TXB2 excretion levels and the 11-dehydro-TXB2:2,3-dinor-6-keto-PGF1α excretion ratio were higher in DS infants than in non-DS infants, irrespective of the presence or absence of PH. Of note is that among the non-DS infants, the urinary 11-dehydro-TXB2:2,3-dinor-6-keto-PGF1α excretion ratio was comparable between the ND-PH group and the ND-C group, whereas both the urinary 11-dehydro-TXB2 level and the 2,3-dinor-6-keto-PGF1α excretion level were elevated in association with PH. This seemingly “compensatory” elevation in the 2,3-dinor-6-keto-PGF1α excretion level in non-DS infants with PH agrees with the experimental findings that TXA2 receptor stimulation upregulates PGI2 biosynthesis [Jeremy et al., 1985] and that shear stress induces the biosynthesis of both TXA2 and PGI2 [Doroudi et al., 2000]. In DS infants with PH, on the other hand, the urinary 2,3-dinor-6-keto-PGF1α excretion level was elevated to a lesser degree than the marked elevation of the urinary 11-dehydro-TXB2 excretion level; thus, the urinary 11-dehydro-TXB2:2,3-dinor-6-keto-PGF1α excretion ratio was also significantly elevated. This observation strongly indicates an imbalance in the biosynthesis of TXA2 and PGI2 that may participate in vasoconstriction and vascular smooth muscle cell proliferation in the lungs.
As for the possible mechanisms by which the presence of an extra copy of chromosome 21 leads to an imbalance in the biosynthesis of vasoactive eicosanoids, we suspect that the presence of an extra copy of the Cu,Zn-superoxide dismutase (SOD) gene on the long arm of chromosome 21 and the overexpression of its protein may account for an imbalance in TXA2 and PGI2 biosynthesis. Increased pulmonary blood flow is known to be associated with the increased biosynthesis of PGH2 from arachidonic acid [Smith et al., 1996; Dubois et al., 1998], which then leads to a balanced increase in both TXA2 and PGI2 biosynthesis. However, in DS infants, both peroxide and peroxynitrate biosynthesis are likely to be up-regulated in the presence of increased SOD activity [De La Torre et al., 1996; Pastor et al., 1998; Pastore et al., 2003]. While peroxide promotes PGH2 biosynthesis [Smith et al., 1996; Dubois et al., 1998], peroxynitrate inhibits the biosynthesis of PGI2, but not TXA2, from PGH2 [Zou and Ullrich, 1996; Zou et al., 1997] consequently, an imbalance in TXA2 and PGI2 biosynthesis arises that eventually leads to vasoconstriction and vascular smooth muscle cell proliferation [Ullrich et al., 2001].
In a previous study examining adult patients with primary (idiopathic) PH and secondary PH arising from collagen vascular diseases, the urinary 11-dehydro-TXB2 excretion level and the urinary 11-dehydro-TXB2:2,3-dinor-6-keto-PGF1α excretion ratio were both elevated [Christman et al., 1992]. In the present study examining pediatric patients, whereas the urinary 11-dehydro-TXB2 excretion level was likewise elevated in non-DS infants with PH, the urinary 11-dehydro-TXB2:2,3-dinor-6-keto-PGF1α excretion ratio was comparable to that in the non-DS control group because of the “compensatory” elevation in the urinary 2,3-dinor-6-keto-PGF1α excretion level. These findings may suggest that the autonomy of the pulmonary vascular endothelial cells is preserved in non-DS infants with PH, which is reversible by cardiac operation, in contrast to the situation in adult patients with irreversible PH.
A previous study by Adatia et al. 1993 on eicosanoid biosynthesis in infants with a left-to-right shunt reported that the ratio of vasoconstrictive eicosanoids to vasodilative eicosanoids was elevated in infants with a left-to-right shunt. In the present study, this ratio was not elevated in non-DS infants with a left-to-right shunt and PH. Although our data could not clarify the reason, two reasons may account for this discordance: first, the subjects in the report by Adatia et al. consisted of both DS infants and non-DS infants. Secondly, about one-half of the infants had PH in the previous study, whereas all the infants in our study had PH.
DS patients are widely known to be at risk for the early development of severe PVOD. Several mechanisms have been proposed, including hypoplasia of the lung (including the vascular bed) [Cooney and Thurlbeck, 1982], sleep apnea resulting in hypoxic vasoconstriction of the pulmonary arteries [Loughlin et al., 1981], and a few specific pathological changes in the pulmonary arteries, including thinning of the media and fibrous intimal proliferation [Yamaki et al., 1983]. Our data indicate that the abnormal biosynthesis of vasoactive eicosanoids in the pulmonary vascular bed may also contribute to the increased vulnerability to PVOD of infants with DS.
One limitation of this study is that the controls were not strictly matched for sex and age with the cases. Nonetheless, a previous study reported that the excretion rates of two of the eicosanoid metabolites that we measured, 11-dehydro-TXB2 and 2,3-dinor-6-keto-PGF1α, did not differ between male and female subjects and were not dependent on age after adjustment for body surface area [Leonhardt et al., 1992]. These findings support the reliability of our results, although a formal case-controlled study would be necessary to obtain more definitive evidence of differences in the biosynthesis of TXA2 and PGI2 in infants with DS and those without DS. In addition, each subject was evaluated only before surgery in the present study. The longitudinal follow-up of individual children would enable the impact of corrective surgery on the balance of TXA2 and PGI2 biosynthesis in DS patients to be elucidated.
In summary, the predominance of TXA2 biosynthesis over PGI2 may lead to vasoconstriction and vascular smooth muscle cell proliferation in DS infants, thereby contributing to the development of PVOD. Of note, such imbalance is observed even in DS infants without congenital heart defect, although its clinical influence is yet to be determined. The increased vulnerability of infants with DS to PVOD may be partly accounted for by this imbalance in the biosynthesis of TXA2 and PGI2.
Over the past 20 years, treatment options for patients with PH have evolved [Humbert et al., 2004], and guidelines for the treatment of PH are being established [Badesch et al., 2004; Galie et al., 2004; Badesch et al., 2007; McLaughlin et al., 2009]. However, the treatment algorithms recommended in guidelines do not yet provide individualized treatment based on an underlying specific condition, for example, Down syndrome. Our data suggest that PGI2 derivatives, such as epoprostenol, may serve as an effective treatment option for PH in patients with DS.
Acknowledgements
We thank the nursing staff of Keio University Hospital for help with the urine collection.




