Hypertrophic cardiomyopathy in a girl with Cornelia de Lange syndrome due to mutation in SMC1A†‡
Giuseppe Limongelli and Silvia Russo contributed equally to this work.
How to Cite this Article: Limongelli G, Russo S, Digilio MC, Masciadri M, Pacileo G, Fratta F, Martone F, Maddaloni V, D'Alessandro R, Calabro P, Russo MG, Calabro R, Larizza L. 2010. Hypertrophic cardiomyopathy in a girl with Cornelia de Lange syndrome due to mutation in SMC1A. Am J Med Genet Part A 152A:2130–2133.
To the Editor:
Cornelia de Lange syndrome (CdLS, OMIM 122470, 300590, and 610759) is a multisystem developmental disorder with an estimated incidence of 1 per 81,000 [Barisic et al., 2008]. Cardiomyopathy has rarely been reported in association with CdLS (three cases of hypertrophic cardiomyopathy (HCM), either isolated or associated with other cardiac defects, out of more than 700 reported patients with CdLS) [Selicorni et al., 2009]. We describe an unusual association between CdLS and HCM in a girl found to carry a mutation in SMC1A, a gene responsible for about 5% of CdLS patients. Since SMC1A has been reported in a very small number of patients (<30) [Liu et al., 2009a], careful description about a rare feature is important to better delineate the phenotypic spectrum.
The patient, a 6-year-old girl with CdLS syndrome, was admitted for cardiovascular examination. There was no family history of congenital heart defects or sudden death. Both the father and the mother had an uneventful clinical history, normal physical examination, ECG, and echocardiogram. The patient had fetal growth retardation from the 3rd month of pregnancy. She was born at term, with low birth weight (1.8 kg, ≪5th centile). Intrauterine growth retardation, development delay, and phenotypic anomalies suggested CdLS at 18 months of age. Frequent episodes of respiratory infections were reported since infancy. She had severe gross motor delay and was unable to crawl and/or walk, requiring a wheelchair at age 4 years. At age 5 years, she was hospitalized for pneumonia. An ECG showed left ventricular hypertrophy. A subsequent echocardiogram revealed a concentric left ventricular hypertrophy.
The following year, she was referred to our department for a complete cardiovascular evaluation and management of HCM. Weight was 12 kg (<3rd centile, corresponding to the 50th centile for 2 years), height 96 cm (<3rd centile, corresponding to the 50th centile for 3.6 years), head circumference 46 cm (<3rd centile, corresponding to the 50th centile for 13 months). Phenotypic examination showed microcephaly, synophrys, downslanting palpebral fissures, long and curly eyelashes, and thin vermilion of the upper lip (Fig. 1A,B). Hand length was 9 cm (<3rd centile corresponding to the 50th centile for 12 months), foot length 12 cm (<3rd centile, corresponding to the 50th centile for 13 months). Developmental milestones were moderately delayed and speech was absent. A 2/6 holosystolic murmur and a third heart sound were heard over the mid-precordium. Echocardiography showed severe, concentric ventricular hypertrophy, with a maximal wall thickness of 15 mm at the anterior interventricular septum (see supporting information Figs. 1 and 2 which may be found in the online version of this article), with a dynamic pressure gradient between the left ventricle and the aorta determining left ventricular outflow tract obstruction at rest (16 mmHg) and after Valsalva maneuver (46 mmHg); mild aortic regurgitation; mitral valve prolapse, with mild mitral regurgitation. ECG showed sinus tachycardia at 160 bpm, with signs of left ventricular hypertrophy.
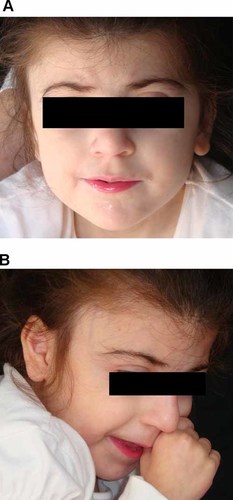
Clinical appearance of the patient (frontal, A; profile, B): synophrys, long eyelashes, and ptosis. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Maternal diabetes and use of steroids during pregnancy were excluded. Metabolic evaluation (blood glucose, blood urea nitrogen, creatinine, electrolytes, lactate, ammonia, creatine phosphokinase, and lactate dehydrogenase investigation) excluded other secondary causes of hypertrophy. A beta-blocker (bisoprolol) was administered, and a follow-up program with regular cardiovascular investigations was drawn for this patient.
Karyotype analysis was normal. Analysis of the major NIPBL gene by DHPLC and direct sequencing showed no mutations. MLPA analysis aimed at ruling out NIPBL intragenic deletions/duplications was negative too. Screening by DHPLC and subsequent direct sequencing of SMC1A (structural maintenance of chromosome-1) gene showed a de novo (parents were negative) point mutation in exon 15 (c.2351T > C) (Fig. 2A). This yet unreported missense change leads to substitution of isoleucine 784, a residue located in the SMC1A coiled-coil 660–1068 arm, with threonine (p.I784T). Multiple alignment of the fragment of SMC1A protein affected by the mutation shows that residue I784 is identical in all analyzed species and belongs to a protein domain highly conserved from Xenopus L. to man (Fig. 2B). The identified sequence change was excluded in 200 chromosomes from healthy Caucasian subjects, thus confirming its pathogenetic role in the associated X-linked CdLS phenotype. A scheme of the gene including our and all SMC1A mutations identified in the literature is reported (Fig. 2C).
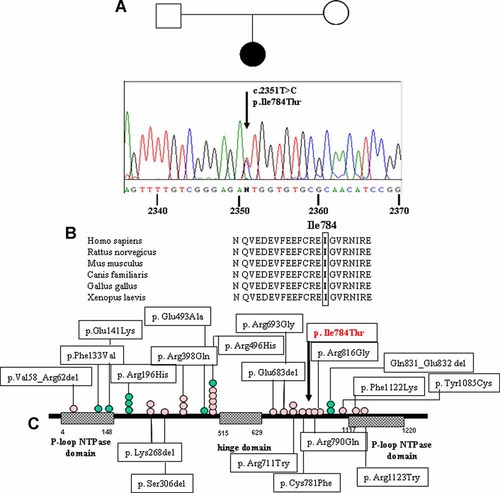
A: Family tree and electropherogram of SMC1A genomic sequence with the arrow indicating the substitution T → C at nucleotide 2351 affecting the amino acid Ile 784; (B) alignment; (C) schematic model of the SMC1A protein indicating the functional domains and the mutations in CdLS patients (blue circles for males and pink for females) reported in the literature. The arrow points to the mutation described in this study. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We described a 6-year-old girl with a relatively mild facial features of CdLS, motor, speech, and neuropsychological delays, and HCM, associated with a de novo SMC1A point mutation (c.2420T > C; p.Ile784Thr). Literature data indicate that SMC1A mutations contribute to approximately 5% of cases of CdLS [Deardorff et al., 2007; Liu et al., 2009a]. They generally result in a consistently mild clinical presentation as compared to NIPBL-mutated patients, with absence of major upper limb defects and structural anomalies, featuring a phenotype that approaches that of apparently nonsyndromic mental retardation [Deardorff et al., 2007; Kline et al., 2007]. Twenty-one mutations in SMC1A have been identified so far among 29 CdLS probands [Musio et al., 2006; Borck et al., 2007; Deardorff et al., 2007; Liu et al., 2009a]. Notably, a predominance of female gender has been observed in patients with SMC1A mutations. It is likely that the mechanism in affected females is due to a dominant-negative effect of the altered protein, and less likely that is due to decreased protein levels or skewed X inactivation, as previously suggested [Musio et al., 2006; Liu et al., 2009a].
The novel mutation herein described c.2420T > C (I784T) maps in exon 15 close to the missense mutation c.2369G > A affecting residue 790 (R790Q) [Deardorff et al., 2007]. The finding of a novel SMC1A missense mutation in this patient is consistent with past reports that all human mutations identified so far in SMC1A are missense or small in frame deletions. However, while most patients with an SMC1A mutation have a mild CdLS phenotype, our patient showed prenatal and postnatal growth restriction along with significant delays. Although congenital heart diseases (such as pulmonic stenosis and atrial septal defect) have been previously reported in patients with SMC1A mutations [Deardorff et al., 2007], HCM with CdLS associated with SMC1A mutation is new. Nevertheless, it is not yet known whether the HCM reported is related to the specific mutation. In familial HCM (OMIM #192600), cardiac hypertrophy is believed to be a “myocite response” to abnormal sarcomeric proteins assembly and function (due to autosomal-dominant mutations in sarcomeric protein genes) [Seidman and Seidman, 2001]. Analysis of the mutant SMC1A proteins has indicated that they are likely to produce cohesin complexes although with altered effects on multiple genomic regions, as demonstrated by the peculiar profile of dysregulated gene expression recently observed in SMC1A-mutant human cells [Liu et al., 2009b]. A number of genes are differently expressed in CdLS with respect to healthy controls, including transcripts abundantly expressed in human heart (e.g., the MAP3K5, mitogen-activated protein kinase kinase kinase 5). We hypothesize that gene dysregulation plays a role in determining the cardiac phenotype in patients with CdLS.
It would be worthwhile to test previously reported CdLS/HCM cases for NIPBL and SM1C1A mutation for possible genotype–phenotype correlations aimed at refining the clinical spectrum of SMC1A-mutated cases.




