Paternal uniparental isodisomy of chromosome 6 causing a complex syndrome including complete IFN-γ receptor 1 deficiency†‡
How to cite this article: Prando C, Boisson-Dupuis S, Grant A, Kong X-F, Bustamante J, Feinberg J, Chapgier A, Rose Y, Jannière L, Rizzardi E, Zhang Q, Shanahan CM, Viollet L, Lyonnet S, Abel L, Ruga EM, Casanova J-L. 2010. Paternal uniparental isodisomy of chromosome 6 causing a complex syndrome including complete IFN-γ receptor 1 deficiency. Am J Med Genet Part A 152A:622–629.
Stéphanie Boisson-Dupuis, Audrey Grant, Authors made an Equal Contribution.
Abstract
Mendelian susceptibility to mycobacterial disease (MSMD) is a rare primary immunodeficiency associated with clinical disease caused by weakly virulent mycobacterial species. Interferon gamma receptor 1 (IFN-γR1) deficiency is a genetic etiology of MSMD. We describe the clinical and genetic features of a 7-year-old Italian boy suffering from MSMD associated with a complex phenotype, including neonatal hyperglycemia, neuromuscular disease, and dysmorphic features. The child also developed necrotizing pneumonia caused by Rhodococcus equi. The child is homozygous for a nonsense mutation in exon 3 of IFNGR1 as a result of paternal uniparental disomy (UPD) of the entire chromosome 6. This is the first reported case of uniparental disomy resulting in a complex phenotype including MSMD. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Mendelian susceptibility to mycobacterial disease (MSMD, OMIM# 209950) is a rare primary immunodeficiency (PID) associated with clinical disease caused by weakly virulent mycobacterial species, such as bacille Calmette-Guerin (BCG) vaccines and environmental mycobacteria (EM) [Casanova et al., 1996; Filipe-Santos et al., 2006]. Patients are also susceptible to Mycobacterium tuberculosis [Jouanguy et al., 1997; Picard et al., 2002; Alcais et al., 2005]. With the exception of salmonellosis, which affects about half of affected individuals, other infectious diseases are rarely reported in these patients, although the range of infections identified is increasing [MacLennan et al., 2004; Chapgier et al., 2006; Filipe-Santos et al., 2006]. Up to five autosomal genes (IFNGR1, IFNGR2, STAT1, IL12RB1, and IL12B) and one X-linked (NEMO) MSMD-causing gene have been reported [Filipe-Santos et al., 2006; Vogt et al., 2008]. The first genetic etiology of MSMD was described in 1996, with null recessive mutations in IFNGR1, which encodes the IFN-γ receptor 1 (IFN-γR1) ligand-binding chain [Jouanguy et al., 1996; Newport et al., 1996]. The human IFNGR1 gene is located on chromosome 6q23.3. Two forms of complete IFN-γR1 deficiency have been described, with and without surface expression of the receptor [Dorman et al., 2004]. Complete IFN-γR1 deficiency remains the most severe etiology of MSMD, as it is associated with early-onset MSMD and a poor prognosis [Dorman et al., 2004]. Hematopoietic stem cell transplantation may be curative; however, it is associated with a high rate of graft rejection, probably due to the high levels of circulating IFN-γ [Reuter et al., 2002; Horwitz et al., 2003; Roesler et al., 2004; Chantrain et al., 2006; Rottman et al., 2008; Moilanen et al., 2009]. Up to 90 complete IFN-γ receptor 1 deficient patients have been reported worldwide; most are homozygous, but some are compound heterozygous for null IFNGR1 alleles [Dorman et al., 2004; Filipe-Santos et al., 2006; Storgaard et al., 2006; Muszlak et al., 2007; Noordzij et al., 2007; Okada et al., 2007; Glosli et al., 2008; Lee et al., 2009]. We investigated an Italian boy with MSMD associated with neuromuscular disease, neonatal hyperglycemia and dysmorphic features, who was homozygous for an IFNGR1 mutant allele inherited from his father but not found in his mother.
CLINICAL REPORT
The patient was born in 2002 to nonconsanguineous Italian parents. The birth was induced at 37 weeks and 1 day due to a decrease in fetal movements, oligohydramnios and intrauterine growth retardation (IUGR). Neonatal examination showed major axial hypotonia, ligamentous laxity, campodactyly, club foot, small hands and feet, hypoplasia of the nails of the hands, and cryptorchidism, retrognathia, macroglossia and high palate. At birth, the child presented with respiratory distress, resulting in a need for nasal oxygen therapy for 2 months. Hyperglycemia was detected and the patient received insulin during the first 48 hr of life to control glycemia. At the age of 2 years, the patient presented with high fever daily over a period of 3 months, accompanied by hepatomegaly and cervical and inguinal lymphadenopathy. Computerized tomography scans showed pulmonary hilum and intra-abdominal lymph node enlargement. Cervical lymph node biopsy showed granulomatous lymphadenopathy with abscesses suggestive of EM disease, and a positive culture was obtained for Mycobacterium fortuitum. Treatment with antimycobacterial agents (including clarythromycin, rifampin, ethambutol, ciprofloxacin and rifabutin) was initiated and maintained for 15 months.
There was no family history of muscular dystrophy or mycobacterial disease and the patient was not vaccinated with BCG. It was hypothesized that the patient had MSMD in the context of a complex developmental syndrome. The patient remained asymptomatic until the age of 5 years, when he presented with necrotizing pneumonia. Bronchoalveolar lavage (BAL) samples tested negative for Mycobacterium spp. Gram-positive coccobacilli were found in the BAL sample and a history of recent exposure to soil containing horse feces was reported. Rhodococcus equi was therefore assumed to be the etiologic agent and antibiotic treatment was initiated, leading to successful recovery.
The patient was fed through a nasogastric tube until the age of 5 years due to problems with oral feeding. The muscular hypotonia gradually improved and the patient was able to stand unaided and walk with the assistance of a walking frame from the age of 4 years. At last patient's follow up cognitive development and language skills were appropriate for age 6 years.
The Patient's karyotype as studied by G-banded method was normal. A muscle biopsy at the age of 1 month suggested primary muscle disease (generalized moderate atrophy of type 2 fibers). An electromyogram showed morphological changes to motor units typical of highgrade myogenic disease. Creatine kinase levels were high at the age of 1 month (243 U/L), but had normalized by the next assessment at the age of 2 months. At the age of 2 years the patient had inconclusive laboratory findings such as leukocyte counts, serum immunoglobulins and complement components C3, C4, and CH50. Mitogen-driven T-cell proliferation was normal. The patient was seronegative for HIV. The IL12-23/IFN-γ circuit was evaluated to search for MSMD etiologies.
We collected blood samples from the patient and his parents after obtaining informed consent. Blood cells from the patient responded to BCG + IL-12 and produced IFN-γ, but did not secrete IL-12p40 and IL-12p70 in response to stimulation with BCG + IFN-γ (Figs. 1A–C). Plasma IFN-γ level was 104 pg/ml. These results were suggestive of complete IFN-γR1 or IFN-γR2 deficiency [Feinberg et al., 2004]. We then cultured Epstein Barr virus-transformed B lymphocytes (EBV-B), as previously described [Dupuis et al., 2003].
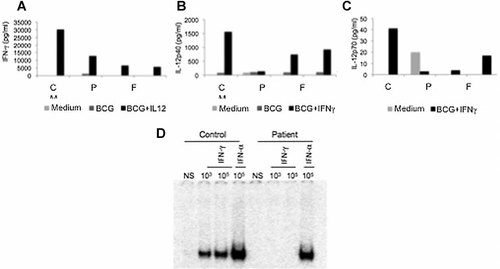
IL12-23/IFN-γ circuit evaluation. Cytokine production of whole blood cells from the patient (P), his father (F) and mother (M), and a healthy control (C), his father and mother, and a healthy control. ELISA data for blood after 48 hr of activation. A: IFN-γ production at 48 hr. B: IL-12p40 production at 48 hr. C: IL-12p70 production at 48 hr. D: IFN-γ receptor-mediated cell responses evaluated by electrophoretic mobility shift assay with nuclear extracts (5 µg) from EBV-B cells from one healthy control and the patient not stimulated or stimulated for 30 min with 103 and 105 IU/ml IFN-γ or 105 IU/ml IFN-α. The results shown are representative of two independent experiments.
Genomic DNA was extracted from leukocytes and EBV-B cell lines and IFNGR1 exons were amplified and sequenced [Dupuis et al., 2003]. The known sequences from Ensembl (http://www.ensembl.org) were aligned by CLUSTALW multiple sequence alignment (http://align.genome.jp). We identified an apparently homozygous nucleotide substitution at position 13840 in exon 3, resulting in a new nonsense mutation (Y113X) upstream from the transmembrane domain of IFN-γR1 (Fig. 2A). These results suggested a diagnosis of recessive, complete IFN-γR1 deficiency. We assessed the IFN-γ response of the patient's EBV-B cells by carrying out an electrophoretic mobility shift assay [Dupuis et al., 2000] in which these cells were stimulated with IFN-γ, using a gamma activating sequence (GAS) probe. EBV-B cells from the patient presented a complete deficiency of GAS-binding proteins after IFN-γ stimulation, as shown by comparisons with control cells, but were able to respond to IFN-α (Fig. 1D). These results suggested that the patient had complete IFN-γR1 deficiency, due to a new, apparently homozygous nonsense mutation in exon 3. However, intrafamilial segregation of the mutant allele of the IFNGR1 gene showed that the patient's father was heterozygous for the Y113X mutation, whereas his mother carried only the wild-type allele of IFNGR1 (Fig. 2).
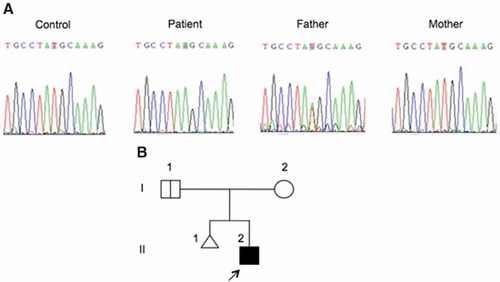
Mutation in the IFNGR1 gene. A: Analysis of the genomic sequence of the IFNGR1 gene. The patient carries a T > A mutation in exon 3, leading to a nonsense mutation (Y113X). The father is heterozygous for the same mutation and the mother presents the wild-type allele. All sequences are shown in forward orientation. B: Pedigree of the family. The proband is represented by a black symbol and indicated by an arrow.
In order to further investigate the mode of inheritance of the Y113X mutation, we genotyped the patient and his parents with the genome-wide SNP 6.0 array (Affymetrix, Santa Clara, CA) including 909,622 SNPs. Genotyping was highly successful, with call rates of 99.0%, 98.8%, and 98.9% in the patient, father, and mother, respectively. In particular, the call rate for the patient on chromosome 6 (out of 56,400 SNPs) was 98.8%, with a proportion of no calls equally distributed over the chromosome (Fig. 3C). Examination of the genotypes at the chromosome 6 of the patient shows an overall loss of heterozygozity, without heterozygous calls along the whole chromosome (Fig. 3B), a pattern that was not observed on any other chromosomes, as exemplified in Figure 3A with chromosome 1. Examination of mother-patient transmissions showed that all maternal chromosomes except chromosome 6 were compatible with a Mendelian inconsistency rate of <0.004, while chromosome 6 displayed a considerable rate of Mendelian inconsistencies (0.172), which were equally distributed over chromosome 6 (Fig. 3C). These results confirm that the mother is indeed the biological mother of the patient, although she did not transmit any part of her chromosome 6 to her child. Examination of father-patient transmissions showed that all paternal chromosomes, including 6, are compatible with the patient with Mendelian inconsistencies rate <0.006. As well as for the mother, Mendelian inconsistencies of paternal origin were equally distributed on chromosome 6 (Fig. 3C). All these data strongly support the view that both copies of chromosome 6 of the patient were inherited from the father, characterizing a paternal UPD of the whole chromosome.
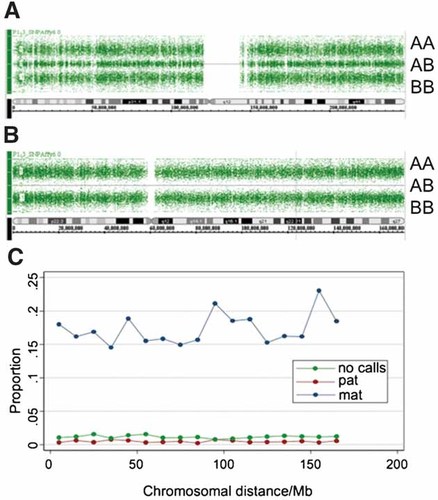
SNP 6.0 array analysis of patient's chromosome 6. Schematic representation of the genotype calls (denoted as AA, AB and, BB) for the SNPs along the entire chromosomes 1 (Panel A) and 6 (Panel B). Chromosome 1 presents a typical distribution of AA, AB, and BB genotypes while no heterozygous AB calls are observed for chromosome 6. Panel C presents the proportion of errors observed along the entire chromosome 6 of the patient for mother-patient transmissions (blue line), father-patient transmissions (red line), and genotype calls (green line).
DISCUSSION
UPD is defined as the inheritance of a pair of homologous chromosomes from a single parent [Engel, 1980]. If the two chromosomes are identical, the UPD is described as isodisomic; if the two chromosomes are not identical, the UPD is described as heterodisomic UPD. It is associated with two main types of developmental risk: the inheritance of a recessive trait and the occurrence of imprinting disorders. The clinical phenotype for an autosomal recessive disorder depends on the gene mutated. Most of the known imprinted genes are critical regulators of growth and development [Fowden et al., 2006; Hurst and McVean, 1997]. To date, up to three PIDs resulting from autosomal recessive disorders due to UPD have been reported: Chediak–Higashi syndrome (OMIM# 214500; chromosome 1), cartilage-hair hypoplasia (OMIM# 250250; chromosome 9) and familial hemophagocytic lymphohistiocytosis (OMIM# 603553; chromosome 10). To our knowledge, UPD has never previously been associated with MSMD. Our patient with UPD6 presented with MSMD, neonatal hyperglycemia, muscular disease and dysmorphic features.
The duplicated chromosomes harboring a mutant IFNGR1 gene account for the recessive form of MSMD in this child despite the presence of the heterozygous mutation in only one of his parents. The recessive nonsense mutation in exon 3 gave rise to a premature stop codon and complete IFN-γR1 deficiency. The severe clinical features of MSMD in our patient are consistent with those of other IFN-γR1-deficient patients [Dorman et al., 2004], with an early onset of symptoms and disseminated infection with rapidly growing mycobacteria, M. fortuitum in this case. Three years after the initial onset of symptoms, the patient presented with necrotizing pneumonia thought to be caused by R. equi, an emerging pathogen of humans [Weinstock and Brown 2002] that had not previously been identified in IFN-γR1-deficient patients. With the exception of salmonellosis [Dorman et al., 2004], few infections have been described in patients with complete IFN-γR1 deficiency. Those detected in other patients include cytomegalovirus [Dorman et al., 1999; Cunningham et al., 2000], human herpesvirus 8 [Camcioglu et al., 2004], Listeria monocytogenes [Roesler et al., 1999], and Histoplasma capsulatum [Jouanguy et al., 1999; Zerbe and Holland, 2005] infections. However, each of these infections occurred in only one to three patients, making it difficult to draw definitive conclusions concerning the extent to which these infections truly reflect genetic predisposition. R. equi is a Gram-positive facultative intracellular pathogen that infects foals, cattle and wild birds, among other animals [von Bargen and Haas 2009]. Foals display a defect in IFN-γ production at birth and there is evidence that the immune response to R. equi depends on the production of IFN-γ [Hines et al., 1997; Giguere et al., 1999; Breathnach et al., 2006]. R. equi is a rarely documented pathogen in human populations [von Bargen and Haas 2009]. R. equi was first described as a human pathogen in 1967, in a patient on immunosupressive treatment for autoimmune hepatitis [Golub et al., 1967]. Most cases of human R. equi infection involve patients taking immunosuppressive treatment or infected with HIV [Arya et al., 2004; Corti et al., 2009; von Bargen and Haas 2009]. The most common presentation of R. equi in immunodeficient patients is necrotizing pneumonia, with or without sepsis [Sistla et al., 2009]. One patient suffering from chronic granulomatous disease presented pneumonia caused by R. equi [Soler-Palacin et al., 2007]. Our patient's history suggests that R. equi may be a potential pathogen in patients with IFNγ-R1 deficiency, and even perhaps in patients with other genetic etiologies of MSMD.
The first UPD6 conferring a recessive disorder was reported in 1990 as a recessive deficiency of the C4 component of the complement system in a girl with systemic erythematous lupus [Welch et al., 1990]. Imprinting disorder due to paternal UPD6 affecting the 6q24–q25 region leads to transient neonatal diabetes (OMIM# 601410). Six published cases of five recessive disorders resulting from either entire or segmental UPD6 have been described: complete C4 deficiency, congenital adrenal hyperplasia, methylmalonic acidemia and agenesis of pancreatic beta cells progressive degenerative disease due to mutation in SCA17 gene [Abramowicz et al., 1994; Kotzot, 2008]. Our patient is the first reported case of IFN-γR1 deficiency in which the recessive disorder results from UPD6. Reported cases of UPD6 not involving recessive disorders have highly heterogeneous phenotypes, ranging from an absence of symptoms to growth retardation, developmental retardation and organ dysgenesis [Kotzot, 2001]. Two genes on human chromosome 6 are known to be paternally imprinted (PLAGL1 and HYMAI), two others are predicted to be paternally imprinted (MRAP2 and BRP44L), and two more are predicted to be maternally imprinted (FAM50B and BTNL2) [Luedi et al., 2007] (www.geneimprint.com). Only PLAGL1, located in the 6q24–q25 region, has been associated with a clinical disorder, transient neonatal diabetes mellitus (TNDM; OMIM# 601410). Twenty-three patients with segmental or complete paternal UPD6 without autosomal recessive disorders have been reported and are listed in Table I [Temple et al., 1995; Bittencourt et al., 1997; Whiteford et al., 1997; Gardner et al., 1998; Christian et al., 1999; Smith et al., 1999; Cave et al., 2000; Das et al., 2000; Hermann et al., 2000; Marquis et al., 2000; Eggermann et al., 2001; Milenkovic et al., 2001, 2006; Valerio et al., 2001; Bonet Alcaina et al., 2002; Bryke et al., 2004; Cockwell et al., 2006; Diatloff-Zito et al., 2007; Felix et al., 2007; Aviram et al., 2008; Kenny et al., 2009]. The abnormal findings common to these patients may be attributed to imprinting disorders. Most of these patients (n = 21) developed TNDM, with and without dysmorphic features. One patient was asymptomatic, with UPD of the entire chromosome 6 identified during HLA typing to identify potential donors for bone marrow transplantation for β-thalassemia major [Bittencourt et al., 1997]. Our patient presented at least seven of the features reported in paternal UPD6 patients: IUGR, macroglossia and other craniofacial dysmorphisms, small hands and feet, club foot, muscular hypotonia and motor retardation. The precise period during which TNDM may be considered to occur has not been precisely defined [Shield, 2000; Barbetti, 2007]. Our patient's neonatal hyperglycemia may therefore be considered to be consistent with this well characterized paternal UPD6 imprinting disorder.
| ID | Karyotype | UPD6 | TNDM | IUGR | Craniofacial dysmorphisma | Abdominal wall defectsb | Bone and joints defectsc | Muscular hypotonia | Motor retardation | Heart defectd | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 47,XY | P (I) | x | x | x | x | x | x | This study | |||
| 1 | 47,XXX | P (I) | x | x | x | x | x |
Valerio et al. 2001 |
|||
| 2 | 46,XY | P (I) | x | x | x |
Milenkovic et al. 2001 |
|||||
| 3 | 46,XX | P (I) | x | x |
Cave et al. 2000 |
||||||
| 4 | 46,XY | P (I) | x | x | x | x | x |
Christian et al. 1999 |
|||
| 5 | 46,XX | P (I) | x | x | x |
Bonet Alcaina et al. 2002 |
|||||
| 6 | 46,XX | P (I) | x | x |
Hermann et al. 2000 |
||||||
| 7 | 46,XX | P (I) | x | x |
Gardner et al. 1998 |
||||||
| 8 | 46,XX | P (I for 6q → 24) | x | x | x |
Das et al. 2000 |
|||||
| 9 | 46,XX | P (I for p12; H for q24-24.3) | x | x | x |
Aviram et al. 2008 |
|||||
| 10 | 47,XXY | P (H) | x | x |
Felix et al. 2007 |
||||||
| 11 | 46,XX | P (I; H for 6q24) | x | x | x |
Milenkovic et al. 2006 |
|||||
| 12 | Not informed | P (I)e | x | x | x | x | x | x |
Diatloff-Zito et al. 2007 |
||
| 13 | Not informed | P (I)e | x |
Diatloff-Zito et al. 2007 |
|||||||
| 14 | 46,XX | P (I) | x |
Marquis et al. 2000 |
|||||||
| 15 | 46,XX | P (I) | x |
Temple et al. 1995 |
|||||||
| 16 | 46,XX/47XX,+r(6) | P (I for 6q23 → qter) | x |
Temple et al. 1995 |
|||||||
| 17 | 46,XY | P (I) | x |
Whiteford et al. 1997 |
|||||||
| 18 | 46,XX | P (I) | x |
Kenny et al. 2009 |
|||||||
| 19 | Not informed | P (I) | x |
Eggermann et al. 2001 |
|||||||
| 20 | 46,XX | P (I)f | x | x |
Bryke et al. 2004 |
||||||
| 21 | 46,XY | P (I for 6q23 → qter) | x | x |
Smith et al. 1999 |
||||||
| 22 | 46,XX | P (I) | x | x |
Cockwell et al. 2006 |
||||||
| 23 | 46,XX | P (I) |
Bittencourt et al. 1997 |
- P, paternal origin. I, isodisomy. H, heterodisomy.
- a Macroglossia as the main symptom (n = 10).
- b Umbilical hernia (n = 3) and exomphalos (n = 1).
- c Small hands and feet (n = 1), club foot (n = 2) and delayed bone maturation (n = 1).
- d Septal defects (n = 2) and mitral valve insufficiency (n = 1).
- e Only markers for the region 6q24 were considered in this patient.
- f Genome-wide UPD. Patient 8 died from neonatal sepsis. Patients 13 and 14 were studied specifically for UPD in the region 6q24. Patient 17 presented with mild, unspecified dysmorphic features. Patient 19 developed cholestasis, due to a paucity of interlobular bile ducts. Patient 21 presented genome-wide UPD with symptoms of UPD 11, 14, 15, and 20 in addition to UPD6. Patient 23 died in the uterus. Patient 24 is asymptomatic.
Our patient also presented with several features not previously reported in paternal UPD6 patients: cryptorchidism, hypoplasia of the nails on his hands, campodactyly, kyphosis and prominent heels. The clinical phenotype of our patient, except for the symptoms related to MSMD, probably resulted from the disruption of imprinting balance. However, we cannot exclude the possibility of another recessively inherited mutation contributing to some phenotypes, such as the muscle disease.
Acknowledgements
We thank Alexandre Bolze Etienne Patin and Arina Samarina for helpful discussion. We thank Ron Liebman, Tatiana Kochetkov, Erin Kirk, Yelena Nemirovskaya, Brooke Delaney, Martine Courat, Tony Leclerc, and Guy Brami for technical and secretarial assistance and all members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions. This project was supported by The Rockefeller University Center for Clinical and Translational Science grant number 5UL1RR024143-03, The Rockefeller University and Robert A. Good and Jeffrey Modell Foundation Fellowship for Primary Immunodeficiency and Immune Reconstitution.




