Periventricular nodular heterotopia and distal limb deficiency: A recurrent association†
How to cite this article: de Wit MCY, de Coo IFM, Schot R, Hoogeboom AJM, Lequin MH, Verkerk AJMH, Mancini GMS. 2010. Periventricular nodular heterotopia and distal limb deficiency: A recurrent association. Am J Med Genet Part A 152A:954–959.
Abstract
Malformations of cerebral cortical development, in particular periventricular nodular heterotopia (PNH), and distal transverse limb deficiency have been reported as associated congenital anomalies. Patients with PNH and transverse limb deficiency can be classified as having amniotic band sequence or Adams–Oliver syndrome (AOS). Controversy exists whether these should be considered separate entities. In some AOS patients, autosomal recessive inheritance has been shown, but in most patients causes are unknown, and both environmental and genetic factors have been implicated. We present three patients with PNH and distal transverse limb deficiency to support the hypothesis that these should be considered part of one group of disorders, and highlight the variable severity of the clinical and neuroradiological phenotype. Chromosome abnormalities were excluded by copy number analysis on 250K SNP microarray data.Research done on limb deficiency as on PNH caused by mutations in known genes, suggests the involvement of vascular developmental pathways. The combination of limb deficiency and PNH may have a common causative mechanism. Recognition and grouping of patients with this combination of abnormalities will help elucidating the cause. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Malformations of cerebral cortical development (MCD) such as periventricular nodular heterotopia (PNH), or polymicrogyria (PMG) can occur as an isolated malformation or in combination with a diverse range of congenital abnormalities [de Wit et al., 2008]. One reported association is with limb anomalies. A combination of limb deficiency and PNH has also been described in the amniotic band sequence (OMIM 217100) and Adams–Oliver syndrome (AOS, OMIM 100300). Both diagnoses have even been suggested in the same patient, highlighting the overlapping findings [Ruggieri et al., 2007; Brancati et al., 2008]. Amniotic band sequence refers to congenital disruption with distal limb deficiencies and constriction rings, associated with other congenital anomalies [Robin et al., 2005]. It remains controversial whether these are caused by external constriction by amniotic bands or by genetic factors that cause both the congenital anomalies and the formation of amniotic bands. AOS is a variable syndrome of scalp defects and distal limb deficiencies. The severe variant with central nervous system involvement is thought to be autosomal recessive [Amor et al., 2000; Brancati et al., 2008; McGoey and Lacassie, 2008]. Other patients with PNH and limb deficiencies are not classified as AOS or amniotic band sequence [Parrini et al., 2006]. In this report we focus on malformations of cortical development, particularly PNH, associated with distal transverse limb deficiency and suggest these may have a shared pathogenesis. To support this, we present three patients with PNH and distal limb deficiency.
PATIENTS
Patient 1
This boy was born to nonconsanguineous parents at 32 weeks of gestation after an uneventful pregnancy. At birth defects of the limbs were noted and he was diagnosed with amniotic band sequence. The left hand is normal, but with a ring constriction at the wrist. Radiographs are normal. The right hand has a normal thumb. There is cutaneous syndactyly, partial between 2 and 3, 4 and 5, and complete between 3 and 4, and a lack of finger creases. There are nails present on digit 2, the fused digits 3–4 and digit 5. Radiographs show a normal thumb and normal metacarpals. Digits 2–5 lack phalanges. Digits 2 and 4 have fused phalanges, digits 3–5 hypoplastic proximal phalanges. The left foot shows short toes 1–3 without nails, with partial cutaneous syndactyly of toes 1 and 2, and complete syndactyly of toes 2 and 3. Toes 4 and 5 appear normal. Radiographs show absence of the distal phalanx of digits 1, 2, and 3, hypoplasia of the middle phalanges of digits 2 and 3, and fusion of the middle and distal phalanges of digits 4 and 5. The right foot also shows short toes 1–3 without nails, and partial cutaneous syndactyly between toes 2 and 3. Radiographs show hypoplasia of the distal phalanx of digits 1 and 3, and absence in digit 2. There is fusion of the middle and distal phalanges of digits 4 and 5 (Figs. 1A and 4A). The patient developed intractable epilepsy at age 19. Brain MRI showed bilateral symmetric occipital PNH (Fig. 2A). Psychomotor development was normal, and he finished highschool. There are no facial anomalies. Occipitofrontal circumference is normal. He has amblyopia of the left eye with divergent strabismus. Neurological findings are normal.
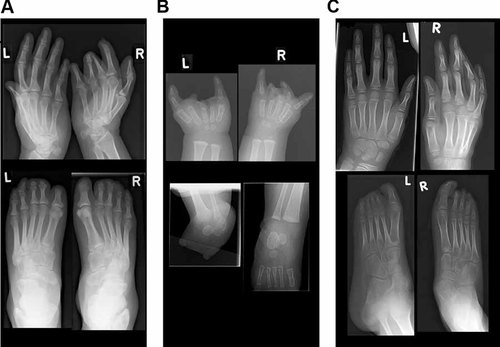
Radiographs of hands and feet. A: Patient 1 at 39 years. Upper panel: normal left hand and transverse limb deficiency of the right hand. Lower panel: feet with deficiencies on both sides. B: Patient 2 at 3.5 months. Upper panel: deficiencies of both hands. Lower panel: absence of metatarsal bones and toes of the left foot, and a normal right foot. C: Patient 3 at 9 years. Upper panel: normal hands. Lower panel: deficiencies of the left foot and a normal right foot.
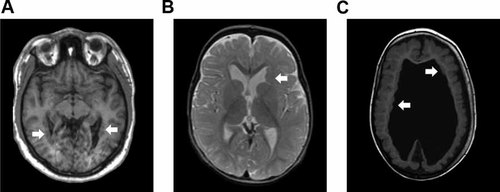
Brain MRI of all patients. A: Patient 1 at age 39 years, T1 weighted image. Note bilateral PNH in the occipital horns of the lateral ventricles (arrows). B: Patient 2 at age 11 months, T2 weighted image. Note a large PNH in the frontal horn of the left lateral ventricle (arrow). Ventricles are mildly enlarged for age. C: Patient 3 at age 3 years. T1 weighted image. Note bilateral PNH (arrows), hydrocephalus and diffuse polymicrogyria. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 2
This girl was born at term by caesarean section, which was prompted by fetal distress. She was dysmature weighing 1,900 g, which was attributed to maternal cannabis, oxazepam, and tramadol use and cigarette smoking. These substances are not reported to cause MCD or limb defects. She has distal deficiencies of three limbs (Fig. 4B). On the left hand digits 2, 3, and 4 are absent. The left thumb is normal, and the fifth finger is short. Radiographs show a normal thumb, lack of the middle or distal phalanx of the fifth finger, short metacarpals 2, 3, and 4 and absence of all phalanges of digits 2, 3, and 4 (Fig. 1B). On the right hand there is a normal thumb and short fifth finger. Digit 2 is hypoplastic with a normal nail. Digits 3 and 4 are rudimentary with ring constrictions, and have no nail. Radiographs show normal metacarpals and thumb. The fifth finger shows no (ossification of the) middle phalanx, and is otherwise normal. Digit 2 shows a delta-shaped proximal phalanx, rudimentary ossification of the middle phalanx, and a hypoplastic distal phalanx. Digit 3 shows rudimentary ossification of the proximal phalanx and absence of other bones. Digit 4 shows a hypoplastic proximal phalanx. The left foot is short with a bud for digits 1 and 5 and dimples at the location of the other digits. Radiographs show absence of metatarsal bones and toes. On the right, there is a clubfoot with cutaneous syndactyly between 2 and 3, otherwise normal toes and nails (Fig. 4B). Radiographs are normal (Fig. 1B). Minor facial anomalies include bilateral epicanthic folds and telecanthus, upward slant of the palpebral fissures, small bitemporal diameter, and prominent upper lip (Fig. 3A). Brain MRI shows a large PNH at the left anterior ventricle (Fig. 2B). She had not developed epilepsy up to the present age of 2 years. Repeated EEGs were normal. Psychomotor development is mildly delayed, and she recently acquired walking. OFC grows at −0.5 SD. On neurological exam she was found to have bilateral abducens nerve palsy and symmetric facial palsy, compatible with Möbius syndrome (OMIM 157900). Muscle tone and strength are normal. Tendon reflexes are symmetrically normal and plantar responses are flexor.
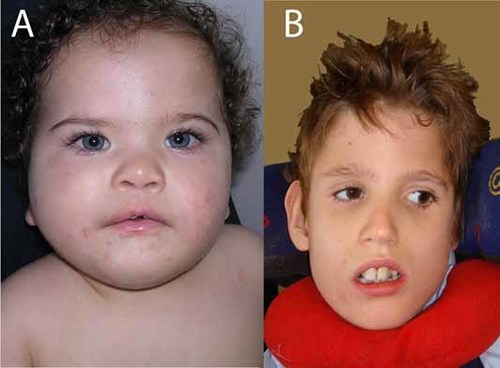
Facial appearance. A: Patient 2 at age 10 months. Note bilateral epicanthic folds and telecanthus, mild upward slant of palpebral fissures, narrow bitemporal diameter, and prominent upper lip. B: Patient 3 at age 9 years. Note bilateral ptosis, short palpebral fissures, bilateral epicanthal folds, and prominent upper central incisors.
Patient 3
This boy was born at term by caesarean, prompted by fetal distress, to nonconsanguineous parents. He had hydrocephalus with OFC at +3.5 SD and received a ventriculo-peritoneal shunt, 1 day after birth. His OFC growth curve dropped to −1 SD over the course of 5 years. At 7 months he developed infantile spasms. MRI showed bilateral PNH with abnormal overlying cortex, consistent with PMG and partial agenesis of the corpus callosum (Fig. 2C). He has limb deficiencies of the left foot with a short first and fourth toes, short toes 2 and 3 with constrictions, all without nails, and normal fifth (Fig. 1C). Radiographs show hypoplasia of the distal phalanx of digit 1, hypoplasia of the proximal phalanx of digits 2–4 and absent middle and distal phalanges of digits 2–5. The right foot shows cutaneous syndactyly between digits 2 and 3, and normal radiographs (Fig. 4C). The hands are normal. Other findings included a supernumerary nipple, bilateral ptosis, short palpebral fissures, and bilateral epicanthal folds (Fig. 3B). He is included in our cohort study of children with MCD [de Wit et al., 2008]. He is now 8 years old and has severe psychomotor retardation with intractable epilepsy, spastic tetraplegia, and scoliosis.
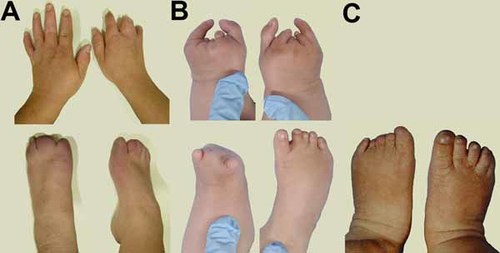
Limbs. A: Patient 1 at age 39 years. Upper panel: normal left hand and cutaneous syndactyly on the right hand. Lower panel: feet show absence of nails on digits 1–3, and cutaneous syndactyly. B: Patient 2 at age 3 months. Upper panel: absence of digits 2, 3, and 4, and short fifth finger on the left hand. On the right hand digit 2 is hypoplastic, digits 3 and 4 are rudimentary with ringconstrictions, and the fifth finger is short. Lower panel: short left foot with a bud for digits 1 and 5 and absence of the other digits. On the right, a clubfoot with cutaneous syndactyly between digits 2 and 3. C: Patient 3 at age 6 months. Upper panel: hands are normal and were not photographed. Lower panel: left foot with short toes 1–4, all without nails. The right foot shows cutaneous syndactyly between digits 2 and 3. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
GENETIC ANALYSIS
Standard G banding karyotyping showed apparently normal chromosomes in all patients. FLNA sequence analysis was done in Patients 1 and 2 with normal results. This was not done in Patient 3 as the MRI phenotype did not fit the FLNA-pattern. DNA-analysis of all patients on Affymetrix 250K NspI SNP arrays, by the CNAG copy number method, did not show any pathogenic chromosome rearrangement. Copy neutral LOH analysis showed several homozygous areas on different chromosomes only in Patient 3, suggesting parental consanguinity.
DISCUSSION
The combination of periventricular nodular heterotopia (PNH) or other cortical malformations and distal transverse limb deficiency is a rare but consistent finding (Table I). PNH and distal limb deficiencies have been reported in patients classified as severe AOS, amniotic band sequence, or with other congenital anomalies. Several case reports describe patients with PNH, limb deficiency, and microphthalmia, cleft palate or midface hypoplasia [Castro et al., 2005; Ruggieri et al., 2007]. The phenotype of our patients overlaps with the severe variant of AOS, although our patients do not have scalp or skin defects [Amor et al., 2000; Brancati et al., 2008; McGoey and Lacassie, 2008]. The extent of the digital defects in our patients is highly variable, as also seen in AOS patients. A combination of PNH with scalp defect without limb deficiency has also been described [Bonioli et al., 2005]. In a recent overview of PNH-phenotypes, four patients (one male) with limb deficiency were included [Parrini et al., 2006]. Limb deficiency has been described with other cortical malformations, but the combination with PNH seems more commonly found (Table I).
| Authors | Sex | PNH | Brain imaging | Scalp/skull defect | Other reported findings |
|---|---|---|---|---|---|
|
Orstavik et al., 1995 (sibs) |
F | − | CT: Periventricular calcification and atrophy | + | Vitreoretinal anomaly |
| M | − | Autopsy: partial agenesis corpus callosum, PVL | + | Vitreoretinal anomaly | |
|
Fryns et al. 1996 |
M | − | CT: porencephalic cyst L hemisphere | + | − |
|
Savarirayan et al. 1999 (sibs) |
M | − | CT: unilateral PMG | + | − |
| F | − | ND | + | Pulmonary valve stenosis. | |
|
Chitayat et al. 1992 |
M | − | CT angiography: abnormal brain vasculature. | + | Bicuspid aortic valve |
|
Romaní et al. 1998 |
M | − | CT: periventricular calcification, wide ventricles | + | − |
|
Mempel et al. 1999 |
M | − | MRI: bilateral PMG | + | Cutis marmorata, cardiac defect |
|
Amor et al. 2000 (sibs) |
M | − | CT: unilateral PMG | + | Cutaneous telangiectasia |
| F | MRI: bilateral PMG | − | Prominent veins, lymphoedema | ||
|
Ünay et al. 2001 |
F | − | CT: periventricular calcification | + | − |
|
Piazza et al. 2004 |
M | − | MRI: periventricular calcification | + | Cutis marmorata, pulmonary hypertension |
|
Patel et al. 2004 (unrelated) |
M | − | MRI: periventricular calcification with infarction, hypoplastic veins | + | Cutis marmorata, cardiac defect |
| M | − | CT: periventricular calcification | + | Cutis marmorata, cardiac defect. Twin pregnancy. | |
|
Parrini et al. 2006 (unrelated) |
M | + | MRI: diffuse bilateral PNHa | − | − |
| F | + | MRI: diffuse bilateral PNH | − | − | |
| F | + | MRI: diffuse bilateral PNHa | − | − | |
| F | + | MRI: diffuse bilateral PNHa | − | − | |
|
Musumeci et al. 2006 (unrelated) |
F | + | MRI: bilateral PNH and agenesis corpus callosum | − | Arthrygryposis, café-au-lait spots |
| F | + | MRI: diffuse bilateral PNH | − | − | |
|
McGoey and Lacassie 2008 (sibs) |
F | − | MRI: partial agenesis corpus callosum, periventricular calcificationa | − | Cutis marmorata prominent veins |
| F | − | CT: periventricular calcificationa | − | Hemangioma face and occiput | |
|
Prothero et al. 2007 |
M | − | MRI: periventricular calcification, wide ventriclesa | + | Falciform retinal folds |
|
Brancati et al. 2008 |
F | + | MRI: diffuse bilateral PNH | − | Prominent veins on trunk |
|
Papadopoulou et al. 2008 |
M | − | MRI: PVL and calcifications | + | − |
|
Balasubramanian and Collins 2009 (sibs) |
M | − | MRI: periventricular calcificationa | − | Cardiac defect |
| F | − | MRI: PVLa | + | − | |
| Current report (unrelated) | M | + | MRI: occipital bilateral PNH | − | − |
| F | + | MRI: unilateral PNH | − | Möbius syndrome | |
| M | + | MRI: bilateral PNH and PMG | − | − |
- PNH, periventricular nodular heterotopia; PMG, polymicrogyria; PVL, periventricular leukomalacia.
- a MRI imaging not published, only description available.
Vascular disruption has been implicated in limb deficiencies, supported by the association with facial clefts, maternal bleeding, twin pregnancies, and other birth defects [Orioli et al., 2003; Taub et al., 2003; Husain et al., 2008]. The pathogenesis of AOS is also thought to be related to vascular problems, such as vascular disruption, abnormal development of small vessels, or abnormal pericyte recruitment to blood vessels [Savarirayan et al., 1999; Amor et al., 2000; Patel et al., 2004]. AOS is associated with other presumably vascular determined congenital defects, such as Poland sequence (OMIM 173800) [Der Kaloustian et al., 1991]. Möbius syndrome, as in Patient 2, can occur with limb defects such as oligodactyly, brachydactyly, syndactyly, polydactyly, or Poland sequence and again abnormal vascular development is implicated [Bavinck and Weaver, 1986; Govaert et al., 1989]. AOS is also associated with cardiac developmental anomalies and cutis marmorata [Zapata et al., 1995].
PNH is a neuronal migration disorder resulting from an inability of early neurons to migrate from the subventricular zone to the cortex. Mutations in the genes coding for filamin A (FLNA, OMIM 30017), and for BIG2 (ARFGEF2, OMIM 605371) have been found in humans with PNH. The most frequent genetic cause of PNH without limb deficiencies, found in approximately 30% of patients, are mutations in the X-linked FLNA gene [Parrini et al., 2006]. In the absence of normal filamin A function, impaired migration of later neurons is due to disrupted cell adhesion and abnormal ventricular ependymal function [Ferland et al., 2009]. FLNA mutations also cause cardiovascular developmental defects and connective tissue abnormalities, and autopsy studies additionally show abnormal glomeruloid microvascular proliferations in the brain in patients with FLNA related PNH [Ferland et al., 2009; de Wit et al., 2009].
PNH is not likely to be directly caused by vascular disruption, but the association with cardiac and vascular anomalies in FLNA patients shows that pathways involved in cell adhesion can affect both the neuro-epithelium and vascular development. Other proteins that regulate axon guidance during brain development, such as Semaphorins, Netrins, Slits, and Ephrins, are also involved in vascular patterning and endothelial cell migration, processes which are essential for limb bud formation [Jones and Li, 2007; Tozer et al., 2007]. Pathways that affect neuronal migration can also affect vascular development, which may increase the chance of prenatal vascular disruption.
Vascular factors have also been implicated in other MCD, mostly polymicrogyria (PMG). Gestational insults, such as cytomegalovirus infection or ischemia, can cause PMG [Marques Dias et al., 1984; de Wit et al., 2008]. Embryonic vascular factors have been implicated in perisylvian PMG with or without limb anomalies in 22q11 microdeletion syndrome [Robin et al., 2006]. Congenital constriction bands with limb deficiencies or AOS are associated with bilateral perisylvian PMG, septo-optic dysplasia and periventricular leukomalacia [Amor et al., 2000; Yamanouchi et al., 2002; Stevens and Dobyns, 2004; Papadopoulou et al., 2008].
In some patients limb deficiencies seem to have resulted from external constriction by amniotic bands; however the recurrent association with cerebral malformation suggests a common, possibly genetic cause in other patients. Our observation suggests that patients with PNH and limb deficiencies manifest abnormalities with a common pathogenesis.
Acknowledgements
We thank for patient care and referral: R.S. Rundervoort, MD, Department of Neurology, Medisch Centrum Haaglanden, Den Haag, the Netherlands, and G.C.B. de Heus, MD, Department of Pediatrics, Erasmus MC Sophia Children's Hospital, Rotterdam, the Netherlands. For DNA analysis: D.J.J. Halley, Department of Clinical Genetics, Erasmus MC, Rotterdam, the Netherlands. For help with the implementation of microarray analysis: P.J. van der Spek, Department of Bioinformatics, Erasmus MC, Rotterdam, the Netherlands.




