Characterization of a new X-linked mental retardation syndrome with microcephaly, cortical malformation, and thin habitus†‡
Christèle du Souich, Athena Chou, and Jingyi Yin contributed equally to this work.
How to cite this article: du Souich C, Chou A, Yin J, Oh T, Nelson TN, Hurlburt J, Arbour L, Friedlander R, McGillivray BC, Tyshchenko N, Rump A, Poskitt KJ, Demos MK, Van Allen MI, Boerkoel CF. 2009. Characterization of a new X-linked mental retardation syndrome with microcephaly, cortical malformation, and thin habitus. Am J Med Genet Part A 149A:2469–2478.
Abstract
X-linked mental retardation (XLMR) affects 1–2/1,000 males and accounts for ∼10% of all mental retardation (MR). We have ascertained a syndromic form of XLMR segregating within a five-generation family with seven affected males. Prominent characteristics include mild to severe MR, cortical malformation, microcephaly, seizures, thin build with distinct facial features including a long and thin face, epicanthic folds, almond-shaped eyes, upslanting palpebral fissures and micrognathia and behavioral problems. Carrier females have normal physical appearance and intelligence. This combination of features is unreported and distinct from Lujan–Fryns syndrome, Snyder–Robinson syndrome, and zinc finger DHHC domain-containing 9-associated MR. We propose the name of this new syndrome to be CK syndrome. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Mental retardation (MR) is generally defined as a significant deficit in intellectual functioning and in adaptive, conceptual, practical, and social skills beginning before the age of 18 years [Luckasson et al., 2002]. Depending on the ascertainment methodology and definition used for MR, its prevalence in the general population is 1–3% in industrialized countries [Roeleveld et al., 1997; Aicardi, 1998; Larson et al., 2001; Ropers and Hamel, 2005]. An Intellectual Quotient (IQ) of <70 is widely regarded as the criterion for the diagnosis of MR and is subdivided according to severity as mild, moderate, severe, or profound [Luckasson et al., 2002]. Additionally, MR has been categorized as syndromic or non-syndromic based on the presence or absence of other clinical, radiological, metabolic, or biological features [Inlow and Restifo, 2004; Chelly et al., 2006].
Despite the high prevalence and morbidity, the physiologic bases of MR remain poorly understood. Identified causes include environmental, epigenetic, and genetic factors [Froyen et al., 2006; Sherr and Shevell, 2006]. At a cellular level, these factors affect neuronal proliferation, migration, arborization, synaptogenesis, or viability [Norman et al., 1995; Sherr and Shevell, 2006; Walsh and Garg, 2006].
Males have an ∼30% higher prevalence of MR than females [Penrose, 1938; Drillien, 1967; Baird and Sadovnick, 1985; McLaren and Bryson, 1987]. This male predominance is explained partially by X-linked mental retardation (XLMR) [Ropers and Hamel, 2005; Raymond, 2006; Chiurazzi et al., 2008], which accounts for ∼10% of MR in males [Kleefstra and Hamel, 2005]. XLMR has been associated with more than 100 loci on the X chromosome [Stevenson et al., 2000; http://www.ggc.org/xlmr.htm].
We report the characterization of a new syndrome associated with XLMR. Affected males have distinct features including an asthenic build, microcephaly, mild to severe MR, seizures, and generalized malformation of the brain cortex. This syndrome is phenotypically distinct from previously described XLMR disorders.
CLINICAL REPORTS
The five-generation family of Russian-Doukhobor descent had no known consanguinity (Fig. 1). All affected males had the same phenotypic presentation (Table I). All unaffected males and carrier females had normal intelligence and physical appearance.
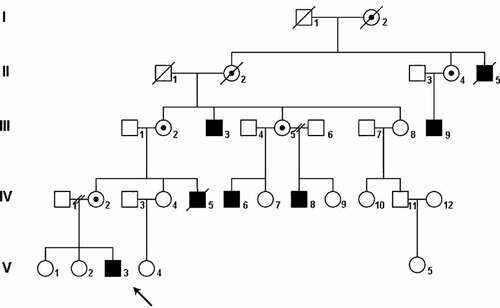
Pedigree of the family. Shaded squares indicate affected males. The propositus is indicated by an arrow. Circles with a dot indicate obligate carrier females. Slashes across circles and squares indicate deaths.
| Clinical Features | Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| V-3 | IV-5 | IV-6 | IV-8 | III-3 | III-9 | II-5a | Total | |
| Cognition and development | ||||||||
| Mild–moderate MR | − | − | − | + | − | + | − | 2/7 |
| Severe MR | + | + | + | − | + | − | + | 5/7 |
| Dysphasia/speech delay | + | + | + | + | + | + | + | 7/7 |
| Dysmorphisms | ||||||||
| Microcephaly (<2 SD) | + | + | + | + | + | n/a | + | 6/6 |
| Long, narrow face | + | + | + | + | n/a | + | n/a | 5/5 |
| Almond-shaped eyes | + | + | + | + | + | + | n/a | 6/6 |
| Epicanthic folds | + | + | + | + | + | − | n/a | 5/6 |
| Upslanting palpebral fissures | + | + | + | + | n/a | + | n/a | 5/5 |
| High nasal bridge | + | + | + | + | n/a | + | n/a | 5/5 |
| High-arched palate | + | + | + | + | + | n/a | n/a | 5/5 |
| Crowded teeth | + | + | + | + | + | n/a | n/a | 5/5 |
| Posteriorly rotated ears | + | + | + | + | n/a | + | n/a | 5/5 |
| Micrognathia/retrognathia | + | + | + | + | n/a | +/− | n/a | 5/5 |
| Malar hypoplasia | + | + | + | + | n/a | + | n/a | 5/5 |
| Long fingers and toes | + | + | + | + | +/− | + | n/a | 6/6 |
| Skeletal findings | ||||||||
| Spine abnormalitiesb | + | + | − | + | + | n/a | n/a | 4/5 |
| Asthenic habitus | + | + | + | + | + | +/− | n/a | 6/6 |
| Ocular findings | ||||||||
| Strabismus | + | + | + | − | + | − | n/a | 4/6 |
| Myopia | + | − | − | − | − | n/a | n/a | 1/5 |
| Bilateral optic atrophy | − | − | − | − | + | n/a | n/a | 1/5 |
| Neurological findings | ||||||||
| Clinical seizures | + | + | + | + | + | + | + | 7/7 |
| Cortical malformation | + | n/a | + | n/a | n/a | n/a | n/a | 2/2 |
| Hypotonia | + | + | + | + | + | n/a | n/a | 5/5 |
| Hyperextensible joints | + | + | + | + | n/a | n/a | n/a | 4/4 |
| Behavioral findings | ||||||||
| Irritability | + | + | + | + | + | − | + | 6/7 |
| Aggression | + | + | + | + | + | − | n/a | 5/6 |
| ADHD | + | n/a | n/a | n/a | n/a | n/a | n/a | 1/1 |
| Autistic behaviors | + | + | + | + | + | + | n/a | 6/6 |
| Cardiac findings | ||||||||
| Heart defectc | + | − | − | − | − | − | n/a | 1/6 |
- +, presence of feature; −, absence of feature; +/−, milder presentation; n/a, information not available; ADHD, attention deficit hyperactivity disorder.
- a This patient died at the age of 2 years and 9 months in 1931. Limited medical records obtained indicated that he was irritable, had severe mental retardation, no words, seizures, and microcephaly.
- b V-3: scoliosis; IV-5: kyphosis; V-8 and III-3: lordosis.
- c V-3: aortic valve stenosis.
Patient V-3
The propositus (Fig. 2A,B) was born at term by spontaneous vaginal delivery following an uneventful pregnancy. His birth weight was 2.75 kg (5–15th centile). He developed seizures in the newborn period. These have included daily multiple brief episodes of unresponsiveness associated with staring and facial and/or limb twitching, and occasional prolonged generalized or secondary generalized convulsive seizures. Electroencephalograms (EEGs) have demonstrated abnormal background and more recently rare epileptiform activity in the left posterior temporal occipital region. His seizures are currently controlled with lamotrigine and clobazam.
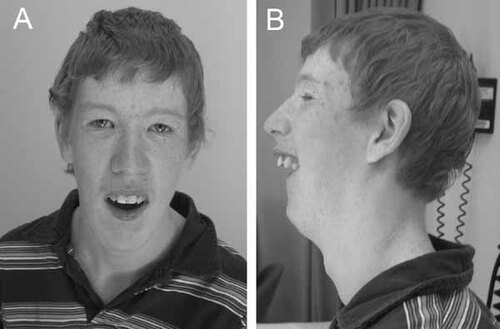
The propositus at age 17 years. His physical features are notable for a long thin face, epicanthic folds, and almond-shaped eyes (A), small jaw, high nasal bridge, and posteriorly rotated ears (B).
A CT scan of the brain at 10 weeks of age showed a diffuse neural migration abnormality suggestive of pachygyria. A brain MRI done at 6 years, 7 months of age demonstrated a diffuse cortical malformation consistent with areas of polymicrogyria and/or pachygyria (Fig. 3A,B).
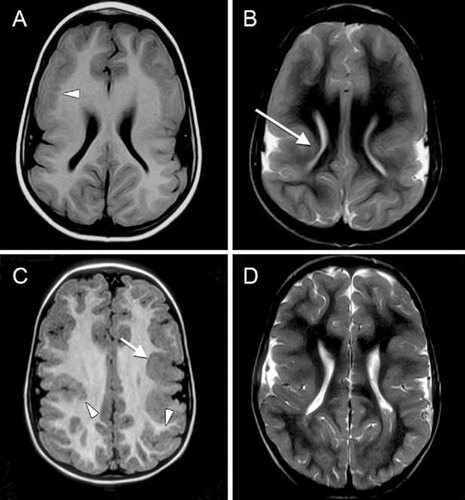
MRI images from the propositus (A,B) and patient IV-6 (C,D). Both cases demonstrate dysmorphic lateral ventricles, simple vertically oriented Sylvian fissures and abnormally thick cortex on T1 (A,C) and T2 (B,D) axial images. The inner margin of the cortex is occasionally irregular, highly suggestive of polymicrogyria (arrow heads), while other regions are smooth and thick, most typical of pachygyria (arrows).
He had markedly delayed developmental milestones; he sat at 17 months and walked at 3 years of age. His first comprehensible word was at 3 years, 8 months of age. Psychoeducational testing at 42 months of age placed his motor skills at the level of a 17-month-old and his communication skills at the level of a 10- to14-month-old. In early childhood, he began manifesting hyperactivity, aggressive behavior, and irritability.
Congenital aortic valve stenosis presented in infancy and required a valvotomy at 6 months of age. Balloon valvuloplasty was done at 18 months and at 9 years. An echocardiogram done at 17 years showed a thickened aortic valve, moderate aortic incompetence, and a mildly hypertrophic left ventricle.
On physical examination at the age of 17 years, his height, weight, and head circumference were 163.1 cm (10th centile), 43 kg (<3rd centile), and 42.9 cm (<2nd centile), respectively. His face was long and narrow with a high nasal bridge and normal philtrum. He had epicanthic folds and almond-shaped eyes with upslanting palpebral fissures (Fig. 2A). The inner canthal distance was 3.2 cm (50–75th centile) and the palpebral fissure length was 3.0 cm (25–50th centile). He had maxillary prognathism, malar hypoplasia, a high-arched palate, a narrow and small mandible, and dental crowding (Fig. 2A,B). His ears were posteriorly rotated and relatively large (75th centile) but not low set (Fig. 2B). He had an asthenic build with relatively long digits, arms, and legs (Fig. 4A–E). His arm span was 167 cm, giving an arm span-to-height ratio of 1.02. His upper segment measured 72 cm and his lower segment measured 91 cm, giving an upper-to-lower segment ratio of 0.79. His middle digit-to-palm ratio was 0.87 (>97th centile). He had normal male genitalia.
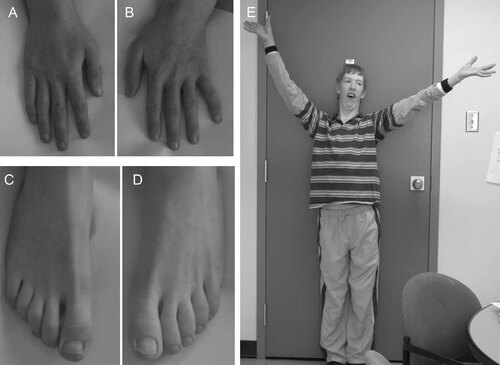
Photographs of the propositus' hands (A,B), feet (C,D), and habitus (E) at 17 years of age. Note the asthenic build, relatively long legs, fingers and toes.
At 17 years of age, he was not fully continent. He had minimal expressive speech (20 words) and used a communication pack to express his needs. He attended high school in a modified program. He was evaluated by a psychiatrist for behavioral concerns including irritability, frequent aggressive outbursts, and sleeping difficulties. He was diagnosed with attention deficit hyperactivity disorder (ADHD), combined type, and insomnia. Treatment with melatonin improved his sleep and quetiapine therapy reduced his aggression.
Results of his laboratory investigations included a normal peripheral lymphocyte karyotype (46, XY at >550 band resolution) and normal array genomic hybridization analysis (SignatureChipOS, Signature Genomic Laboratories, Spokane, Washington). Serum electrolytes, complete blood count, plasma amino acids, acyl carnitine profile, serum lactate, serum biotinidase activity, plasma ammonia, urine oligosaccharides, urine mucopolysaccharides, urine organic acids, urine purines and pyrimidines, urine creatine and guanidinoacetate, and serum sterols were normal. Transferrin showed a normal pattern of glycosylation as assessed by isoelectric focussing. His skeletal survey was not diagnostic of a skeletal dysplasia and his bone age was appropriate. Molecular testing did not identify a FMR1 or ARX repeat expansion or a mutation of MECP2, FLNA, SLC6A8, MED12, GDI1, ZDHHC9, or UPF3B.
Patient II-5
Little medical information was available on this patient. He was born in 1928 and institutionalized at the age of 2 years and 7 months because of severe MR and intractable seizures. He was unable to sit, walk, or talk. He had a small head and was described as irritable. He died at 2 years and 9 months from influenza and seizures.
Patient III-3
This patient was born at term following a normal pregnancy. There were no health problems reported in the neonatal period. He had marked developmental delay apparent within the first year of life. Seizures began in infancy. He was institutionalized at the age of 2 years and upon admission, he was noted to have generalized hypotonia, inability to walk or stand, microcephaly, and bilateral optic atrophy. Other features noted at 62 years of age, included an asthenic habitus with relatively long fingers and toes, strabismus, almond-shaped eyes, epicanthic folds, a high-arched palate, dental crowding, large ears, and marked lordosis. He had severe MR and was non-verbal.
Patient III-9
Based on the limited available information, this patient was the product of a normal pregnancy and delivery. An evaluation at 4 years of age showed that he had speech delay, seizures, and mild to moderate MR. At the age of 17 years, he was described as well-mannered and could speak although he had difficulty forming long sentences. He could dress himself but could not tie his shoelaces. On examination at 18 years of age, he had a long face, almond-shaped eyes, upslanting palpebral fissures, malar hypoplasia, high nasal bridge, and posteriorly rotated ears; he also had long fingers and toes. At 54 years of age, he lived in a supervised home and worked in a sheltered workshop.
Patient IV-5
This patient was born at 43 weeks gestation after an uncomplicated pregnancy. His birth weight was 3.5 kg (50th centile). Throughout infancy and early childhood, he was restless and irritable and had frequent temper tantrums. He was hypotonic and had intractable seizures and severely impaired development, which resulted in his institutionalization at the age of 3 years. Head circumference and height measurements were consistently <3rd centile, and his weight was between the 25th and 50th centile. On physical examination, he manifested a long narrow face with malar hypoplasia, epicanthic folds, almond-shaped eyes, upslanting palpebral fissures, a high arch palate, and dental crowding (Fig. 5D). He had strabismus, which was surgically corrected. At 13 years of age (Fig. 5E) he was described as non-verbal, short tempered, hypotonic, and severely impaired. As an adult (Fig. 5F), he had an asthenic build with long fingers and toes and hypermobile joints in addition to the above features. He died at the age of 39 years from complications of non-Hodgkin's lymphoma.
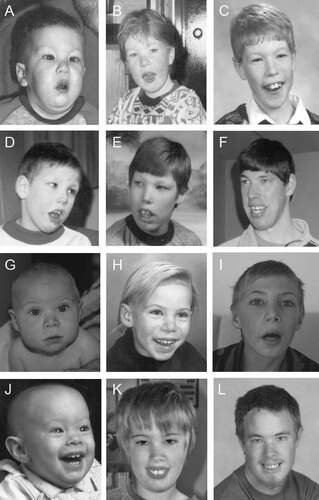
Development of facial features in the propositus and other affected males over time. The propositus (V-3) at ages 18 months (A), 4 years (B), and 8 years (C). Patient IV-5 at ages 6 years (D), 13 years (E), and 30 years (F). Patient IV-6 at ages 6 months (G), 7 years (H), and 10 years (I). Patient IV-8 at 5 months (J), 11 years (K), and 20 years (L). Note the common features of a long face, almond-shaped eyes, epicanthic folds, upslanting palpebral fissures, and high nasal bridge.
Patient IV-6
Patient IV-6 was born at 38 weeks gestation following a pregnancy complicated by maternal toxaemia. His birth weight (2 kg), length (44 cm), and head circumference (29.5 cm) were all <3rd centile. At birth, he had long fingers, a left inguinal hernia, a left thigh lymphangioma, cutis marmorata, and generalized patchy hyperpigmentation. When assessed at age 6 months, he showed almond-shaped eyes, bilateral epicanthic folds, upslanting palpebral fissures, strabismus, malar hypoplasia, posteriorly rotated ears, and a small chin (Fig. 5G). Seizures began in infancy and included sudden body or focal limb jerks. EEG demonstrated abnormal background with bilateral epileptiform activity. Development was globally delayed; he walked at 2 years of age but never developed speech. At 7 age years (Fig. 5H), his face was beginning to elongate and his nasal bridge was becoming more prominent. At 10 years of age (Fig. 5I), he had developed a characteristic asthenic habitus with a long face, long fingers and toes, high-arched palate, and dental crowding. His head circumference was 49.75 cm (<2nd centile), his weight was 25 kg (<3rd centile), and his height was 134.5 cm (10–25th centile). His arm span was 133 cm, giving an arm span-to-height ratio of 0.99. He was illiterate and incontinent and attended a school for intellectually disabled children. Behaviorally, he was aggressive and easily angered. An MRI done at the age of 44 months detected pachygyria and/or polymicrogyria (Fig. 3C,D).
Patient IV-8
This patient was born at term following a pregnancy complicated by maternal toxemia. His birth weight was 2.2 kg (<3rd centile). On examination, he had epicanthic folds, almond-shaped eyes, upslanting palpebral fissures, and posteriorly rotated ears (Fig. 5J). He developed a seizure disorder at the age of 10 months. At 20 months, his head circumference (44 cm) and weight (8.6 kg) were <3rd centile, and his length (81 cm) was at the 10th centile. A developmental assessment at 37 months of age identified mild delays in his fine and gross motor and receptive and expressive language skills. At age 11 years, he was described as short tempered and aggressive. He had an asthenic habitus with long fingers and toes, a long face, a high-arched palate, and dental crowding (Fig. 5K). He graduated from high school in a modified/life skills program. At 20 years of age (Fig. 5L), he had developed a prominent nasal bridge. His head circumference was 51.5 cm (<2nd centile), his weight was 64 kg (25th centile), and his height was 169 cm (10–25th centile). His arm span was 165 cm, giving an arm span-to-height ratio of 0.98. He lived with his parents and was unable to maintain employment.
Patient IV-2
This carrier female was born following an uncomplicated pregnancy. She had normal developmental milestones. On examination at 42 years of age, she had normal intelligence and no health problems. Her height (161.6 cm), weight (56.5 kg), and head circumference (54.5 cm) were all at the 25–50th centile. She had a normal nasal bridge, philtrum, and lips. She had normally shaped eyes with an interpupillary distance of 5.5 cm (25th centile). Her palate and dentition were normal. Her ears were normally formed and positioned but large (+2 SD). She did not have an asthenic build and was not disproportionate. Her arm span was 159 cm, giving an arm span-to-height ratio of 0.98. Her upper segment measured 88 cm and her lower segment measured 73.5 cm, giving an upper-to-lower segment ratio of 1.2. Her middle digit-to-hand length ratio was 0.44 (75th centile). A brain MRI done at 42 years of age was normal. X chromosome inactivation studies showed random inactivation.
Patient III-2
This carrier female was born after an uneventful pregnancy. Developmental milestones were normal. On examination at the age of 63 years, she had normal intellect. Her height was 149.79 cm (<3rd centile), her weight was 80 kg (90–95th centile), and her head circumference was 54.5 cm (25–50th centile). Her face was round with a normal nasal bridge and normal philtrum. She had normally shaped eyes with upslanting palpebral fissures and an interpupillary distance of 5.75 cm (50th centile). She had a normally arched palate, normal mandible, and no dental crowding. Her ears were normally placed and positioned but large (>2 SD). Her arm span was 154 cm, giving an arm span-to-height ratio of 1.03. Her middle digit-to-hand length ratio was 0.46 (>97th centile). She did not have an asthenic build.
Patient III-5
This carrier female was born in 1955 after a normal pregnancy and delivery. She had normal development milestones. On examination at the age of 54 years, she had normal cognition, and a history of type II insulin dependent diabetes and heart disease. Her height was 151 cm (3rd centile), her weight was 73 kg (75–90th centile), and her head circumference was 54 cm (30th centile). She had normally shaped eyes with an interpupillary distance of 6.75 cm (97th centile). She had a normal nasal bridge and philtrum. Her ears were normally shaped and positioned but large (>2 SD). She did not have dental crowding and her palate was normal. Her arm span was 154 cm giving an arm span-to-height ratio of 1.02. Her middle digit-to-hand length ration was 0.44 (75th centile). She did not have an asthenic build.
DISCUSSION
We report a five-generation family of Russian-Doukhobor descent with XLMR and distinctive features in affected males (Table I). Clinical manifestations include mild to severe MR, microcephaly, cortical malformation, seizures, an asthenic build with long fingers and toes, and abnormal behaviors. MRI examinations revealed a diffuse cortical malformation suggestive of polymicrogyria and/or pachygyria in two individuals. Prominent dysmorphic features include a long face, almond-shaped eyes, epicanthic folds, upslanting palpebral fissures, high nasal bridge, high-arched palate with dental crowding, and micrognathia. Behavioral abnormalities consist of irritability, aggression, and autistic-like features.
Based on the X-linked segregation of disease and the clinical features, the differential diagnosis includes Lujan–Fryns syndrome (LFS) (OMIM 309520), Snyder–Robinson syndrome (SRS) (OMIM 309583), and zinc finger DHHC domain-containing protein 9-associated MR (OMIM 300646). Comparison of these syndromes to our patients identified many similarities; however, the differences distinguish this as a previously undescribed syndrome (Table II).
| Clinical features | Frequency of features from the literature | Affected males of this family (n = 7) | ||
|---|---|---|---|---|
| LFSa (n = 28) | SRSb (n = 10) | ZDHHC9c (n = 9) | ||
| Skeletal findings | ||||
| Microcephaly (<2%) | 0/22 | 0/9 | 0/6 | 6/6 |
| Lordosis | 2/12 | NR | NR | 2/5 |
| Scoliosis | 2/12 | NR | 0/1 | 1/5 |
| Kyphoscoliosis | 5/12 | 7/8 | 0/1 | 0/5 |
| Pectus excavatum | 13/24 | 3/8 | 1/3 | 0/4 |
| Osteoporosis | NR | 5/5 | NR | 0/5 |
| Dysmorphisms | ||||
| Long, narrow face | 27/28 | NR | 3/3 | 5/5 |
| Facial asymmetry | NR | 5/9 | NR | 0/5 |
| Almond-shaped eyes | NR | NR | NR | 6/6 |
| Epicanthic folds | NR | NR | NR | 5/6 |
| Upslanting palpebral fissures | 1/13 | NR | NR | 5/5 |
| Posteriorly rotated ears | 6/21 | 0/5 | NR | 5/5 |
| Malar/maxillary hypoplasia | 16/19 | 5/5 | NR | 5/5 |
| High nasal bridge | 25/26 | 3/5 | NR | 5/5 |
| Short, deep philtrum | 18/20 | 3/3 | NR | 0/5 |
| High-arched palate | 24/24 | 3/7 | 0/1 | 5/5 |
| Cleft palate | 0/24 | 2/6 | NR | 0/6 |
| Crowded teeth | 8/10 | NR | NR | 5/5 |
| Thin upper lip | 14/24 | 6/8 | NR | 1/5 |
| Prominent lower lip | NR | 9/9 | NR | 0/5 |
| Micrognathia/retrognathia | 12/22 | NR | NR | 5/5 |
| Cognition and behavior | ||||
| Mild–moderate MR | 17/22 | 6/9 | 8/9 | 2/7 |
| Severe MR | 3/22 | 3/9 | 1/9 | 5/7 |
| Hypernasal speech | 16/22 | 6/6 | NR | 0/6 |
| Behavioral disturbancesd | 19/26 | NR | 1/1 | 7/7 |
| Autistic behaviors | 10/25 | NR | NR | 6/6 |
| Neurological findings | ||||
| Clinical seizures | 2/17 | 3/8 | NR | 7/7 |
| Pachygyria | 0/9 | 0/5 | NR | 2/2 |
| Corpus callosum agenesis | 4/11 | 0/5 | NR | 0/2 |
| Neuromuscular anomaly | NR | 6/7 | NR | 0/6 |
| Ophthalmologic findings | ||||
| Optic atrophy | 0/6 | NR | 0/1 | 1/5 |
| Myopia | 1/6 | 2/3 | 0/1 | 1/5 |
| Strabismus | 0/6 | NR | 0/1 | 4/6 |
| Cardiac findings | ||||
| Heart defect | 4/12 | 1/10 | 0/1 | 1/6 |
- LFS, Lujan–Fryns syndrome; SRS, Snyder–Robinson syndrome; ZDHHC9, zinc finger DHHC domain-containing protein 9-associated mental retardation; NR, not reported.
- a Schwartz et al. 2007, Williams 2006, Lerma-Carrillo et al. 2006, Tarpey et al. 2007, Wittine et al. 1999, De Hert et al. 1996, Gurrieri and Neri 1991, Lujan et al. 1984, Lalatta et al. 1991, Rivera et al. 1992, Fryns and Buttiens 1987.
- b Cason et al. 2003, Arena et al. 1996, de Alencastro et al. 2008. One of the patients reported by Arena et al. 1996 died at the age of 2 weeks. He was said to have a high-arched palate and VSD.
- c Raymond et al. 2007.
- d Behaviors include irritability, aggression, attention deficit hyperactivity disorder, obsessive compulsive disorder, impulse control disorder, shyness, schizophrenia, hallucinations, and anxiety. The most prominent and consistent behavior in our patients was aggression.
LFS is defined by mild to moderate MR, long narrow face, maxillary hypoplasia, long nose with high and narrow bridge, short and deep philtrum, thin upper lip, high-arched palate, micrognathia and retrognathia, low-set posteriorly rotated ears, nasal speech, and generalized hypotonia. Most individuals have a slender habitus with long, thin fingers and toes. Joint hypermobility and pectus excavatum may also be present [Van Buggenhout and Fryns, 2006]. Although the affected males in our family had a similar asthenic build, long and thin face, high-arched palate, and high nasal bridge, they lacked the short deep philtrum of LFS and had features not described in LFS: epicanthic folds, almond-shaped eyes, upslanting palpebral fissures, microcephaly, and seizures. Additionally, brain MRIs of our patients showed a diffuse cortical abnormality, which is not a feature of LFS. Finally, in contrast to the mild to moderate MR seen in LFS, most of our patients are more severely affected and mute. Because of overlapping features, however, molecular testing was done. We did not detect alterations in MED12 [Schwartz et al., 2007], UPF3B [Tarpey et al., 2007], or terminal 5q [Stathopulu et al., 2003], the known genetic causes of LFS.
Although not generally characterized by an asthenic habitus, FG, or Opitz–Kaveggia syndrome (OMIM 305450) deserves mention because it is allelic to LFS and is difficult to diagnose due to its variable expressivity [Lyons et al., 2009]. Distinguishing features of FG syndrome include anal anomalies, severe constipation, broad thumbs and halluces, and abnormalities of the corpus callosum. Affected individuals also have MR, hypotonia, macrocephaly and distinct facial features such as a tall and prominent forehead, frontal hair upsweep and abnormal ears. Several genetic loci have been associated with FG syndrome including mutations in MED12 [Risheg et al., 2007; Lyons et al., 2009], FLNA [Unger et al., 2007], and UPF3B [Tarpey et al., 2007]. The affected males in our family lacked typical features of FG syndrome such as anal anomalies, constipation, macrocephaly, and broad thumbs and halluces. Sequencing of MED12, FLNA, and UPF3B did not detect any mutations in our propositus.
SRS is characterized by a slender body build with a long thin face, prominent lower lip, high-arched palate, and long fingers and toes [Snyder and Robinson, 1969]. Affected males also have diminished muscle bulk, osteoporosis, kyphoscoliosis, MR, hypotonia, unsteady gait, and other non-specific neurological problems [Arena et al., 1996; Cason et al., 2003]. Although our patients presented with some similar facial features as well as an asthenic habitus, they lacked the prominent lower lip, osteoporosis, and kyphoscoliosis.
Raymond et al. 2007 reported four families with XLMR with mutations affecting highly conserved residues in the zinc finger DHHC domain-containing protein 9 (ZDHHC9; Xq26.1). The phenotype of the affected males in three of these families was characterized by a thin habitus, long face and digits, joint hypermobility, and moderate MR. Except for the difference in severity of the MR, this phenotype overlaps with that of our patients. However, sequencing of our proband's DNA did not identify a ZDHHC9 mutation.
We also considered another syndromic XLMR disorder characterized by microcephaly and asthenic build reported by Turner et al. 2003. However, those patients differed from ours in that they had severe infantile hypotonia, and distinctly different facial features.
X-linked cortical malformation disorders including bilateral perisylvian polymicrogyria (OMIM 300388), lissencephaly/subcortical band heterotopia (OMIM 300067), periventricular nodular heterotopia (OMIM 300049), and lissencephaly and abnormal genitalia (OMIM 300215) were also considered. However, the characteristics of the cortical malformation in these syndromes differ from those seen in our patients. Abnormal genitalia as seen in lissencephaly and abnormal genitalia syndrome were not present in our patients. In addition, the prominent dysmorphic features present in our patients are not observed among patients with these cortical malformation disorders [Leventer et al., 2000].
Therefore, to the best of our knowledge, the affected individuals in our family are distinct from all previously reported XLMR and cortical malformation syndromes. The defining characteristics in affected males are an asthenic habitus with distinctive facial features, a diffuse cortical malformation with features of polymicrogyria and/or pachygyria, seizures, mild to severe MR, irritability, and aggression. We propose the name of this new syndrome to be CK syndrome.
Linkage analysis performed in this family identified a 5 MB region on Xq28 segregating with disease. Molecular and biochemical characterization of this syndrome is currently underway [McLarren et al., 2009]. Identifying the disease-associated gene will help elucidate the molecular pathophysiology of this syndrome, aid in the diagnostic evaluation of MR in males in other families, allow investigation into the genotypic and phenotypic spectrum of the syndrome, and ultimately provide insight into potential therapies.
Acknowledgements
The authors thank Dr. Jan M. Friedman, Dr. Keith McLarren, and Ms. Rosemarie Rupps for critical review of this manuscript. This work was supported in part by a British Columbia Children's Foundation Telethon Award (C.D.S.), a Scottish Rite Charitable Foundation Award (C.D.S.), a Child and Family Research Institute Establishment Award (C.F.B.), a Michael Smith Scholar Award in the Biomedical Sciences (C.F.B.) and the Clinical Genomics Platform of the Michael Smith Foundation for Health Research.