Anterior segment anomalies of the eye, growth retardation associated with hypoplastic pituitary gland and endocrine abnormalities: Jung syndrome or a new syndrome?†
How to cite this article: Al-Gazali L, Shather B, Kaplan W, Algawi K, Ali BR. 2009. Anterior segment anomalies of the eye, growth retardation associated with hypoplastic pituitary gland and endocrine abnormalities: Jung syndrome or a New Syndrome? Am J Med Genet Part A 149A:251–256.
Abstract
We report on two children from an inbred Arab family with anterior segment anomalies of the eyes, growth retardation, associated with small pituitary gland, and endocrine abnormalities. The features in the sibs in this report are similar to those described in Peters-plus syndrome. However, small pituitary gland associated with growth hormone deficiency has not been reported in Peters-plus syndrome. In addition, sequencing of the B3GALTL gene, the gene implicated in Peters-plus syndrome did not reveal any mutation in the sibs reported here. The association of anterior segment anomalies of the eye, growth retardation, and endocrine problems has previously been described by Jung et al. in 1995. We suggest that the features in the children in this report could represent variable manifestation of this syndrome or previously not described syndrome. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Peters-plus syndrome (OMIM 261540) is a rare autosomal recessive disorder characterized by the combination of anterior segment anomalies of the eye, a typical face, clefting, short limb dwarfism, and developmental delay [Wenniger-Prick and Hennekam, 2002]. The clinical features of this disorder vary considerably making it difficult to make a definite diagnosis in many instances. The molecular basis of this disorder was largely unknown until recently when Lesnik Oberstein et al. 2006 found a 1.5 Mb homozygous deletion on chromosome 13 in two siblings and consequently identified two mutations in B3GALTL gene in 18 patients. B3GALTL gene encodes a putative glycosyltransferase of yet unidentified function. A homozygous point mutation (c1020 + 1G-A) was detected in 16 patients and two patients were heterozygous for this mutation in addition to a point mutation (c.437 + 5G-A) on the second allele [Lesnik Oberstein et al., 2006]. Both mutations led to splice alteration and therefore result in protein truncation and hence loss of function.
In 1995, Jung et al. described two unrelated children with features overlapping Peters-plus syndrome. These included: anterior segment anomalies, proportionate short stature, and developmental delay. The facial dysmorphic features were different from Peters-plus syndrome and the children had additional features not described in Peters-plus syndrome. For example these children had endocrine abnormalities (growth hormone and thyroid hormone deficiency) and cerebellar malformations. The authors suggested that these children had a new previously not described autosomal recessive syndrome.
We report on two sibs from a consanguineous Arab family with features similar to the syndrome reported by Jung et al. 1995 and to Peters-plus syndrome but with no mutation in B3GALTL gene.
CLINICAL REPORT
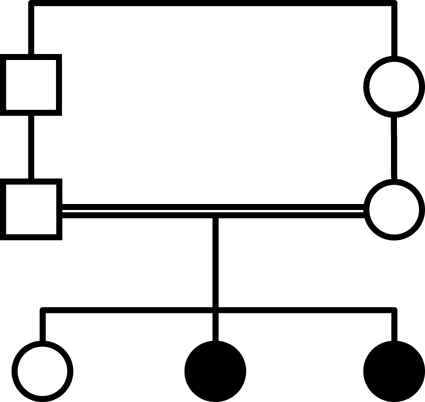
The parents are first cousins of Syrian origin. They have three girls, two of them are affected. There was no family history of any genetic problem and the parents were normal.
Patient 1, this child was the product of normal pregnancy and delivery. Her birth weight was 2.4 kg, length 40 cm and head circumference 31 cm (All below the 10th centile). There were no neonatal problems apart from hypoglycemia which was treated with glucose infusion. In the first year of life she was noted to have bilateral corneal opacification, dysmorphic features, and growth retardation. She had a systolic murmur and echocardiography revealed pulmonary stenosis. A brain MRI showed a very small pituitary gland and endocrine studies indicated growth hormone deficiency. She was started on growth hormone injections. She had bilateral corneal graft and left cataract extraction at the age of 3 years. No further information is available as she was born and followed up in Syria.
She was assessed by us at the age of 7 years. Her weight was 9 kg (≪3rd centile), height 82.9 cm (≪3rd centile) and head circumference 45.8 cm (-2 SD). She had several dysmorphic features which included: distinctive angular nose, small mandible, short spindle shaped fingers, small hands, and feet. Her bone age was 5 years at the age of 7 years (Fig. 1). Ophthalmic evaluation showed clear corneal graft in the right eye and dense opacification of the corneal graft in the left eye. She had central, steady and maintained fixation on the right side and un-center unsteady, and un-maintained fixation of the left eye with occasional fixation to light (indicating poor left vision).

Patient 1 at the age of 7 years. Note corneal opacity, distinctive nose, and micrognathia.
When assessed by the endocrinologist, height was -8SDS, IGF-I 150 µg/L (N 175–460) and IGF-BP3 2290 µg/L (N 910–3460), both were drawn 3 weeks after the discontinuation of growth hormone treatment. Corrected Ca was 2.43 mmol/L (N 202–2.6) while she was not on calcium supplementation and alkaline phosphatase was 223 U/L. The patient was also found to be vitamin D deficient, 25(OH)D3 39 nmol/L (N 150–150), and was started on ergocalciferol 1000 IU for 3 months and then maintained on 400IU daily. All other endocrine investigations were normal. Growth hormone injections were re-introduced at 0.05 mg/k/d. This resulted in increasing the IGF-I to 333 µg/l, and growth velocity of 6 cm in 13 months. She had mild to moderate developmental delay.
Patient 2 is the sister of Patient 1. She was the product of normal pregnancy and delivery. Her birth weight was 2800 gm (10th centile), length 41 cm (<10th centile) and head circumference was 32 cm (<10th centile). She was noted to have bilateral corneal opacities in the first week of life.
She was assessed by us at the age of 8 months. Her weight was 5.4 kg, length 57 cm, and head circumference 38.3 cm (All below the 3rd centile). She had significant corneal opacities with neovascularization of the cornea (Fig. 2a). The anterior chamber was shallow but details of the anterior segment were not possible due to severe corneal opacities. In addition she had deep-set eyes which were widely spaced, depressed nasal bridge with short wide nose, and a triangular mouth. The proximal segments of the limbs appeared short and her feet were small (Fig. 2b). The patient's fingers were short and spindle shaped. Endocrine studies revealed a height SDS of −5.5 with baseline IGF1 of less than 30 µg/L (N 82–166 mg/L) and IGF binding protein of 1313 µg/L (N 1090–2490). Brain MRI showed hypoplastic corpus callosum indicating, along with the laboratory findings, a possible growth hormone deficiency. She was put on growth hormone injections (0.05 mg/kg/day). Her height was −11 SDS after 13 months of treatment, in spite of the increase in IGF-I to 190 µg/L. X-Ray of the extremities revealed no deformities, and thyroid test and bone profile (Ca, alkaline phosphatase, 25(OH)D, and PTH) were all normal.
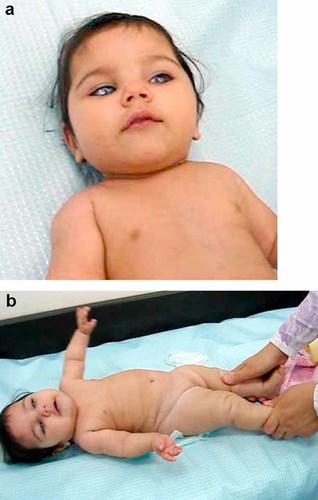
Patient 2 at the age of 1 year of age. a: Note bilateral corneal opacity, depressed nasal bridge, and round face. b: Note small hands and short limbs. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
She was hypotonic and had moderate delay in her developmental milestones. Echocardiography was normal. She underwent successful bilateral penetrating keratoplasty with significant improvement in her vision.
DNA Sequence Analysis
DNA sequence analysis of the B3GALTL gene from the affected children did not show any mutation. In fact B3GALTL gene sequence was heterozygous in various positions further confirming lack of involvement of this gene in the development of the phenotype in this family. This assumption is based on the affected children being homozygous at the disease locus.
DISCUSSION
The Peters anomaly is a morphological finding, rather than a distinct entity. The central corneal endothelium is absent with abnormal corneal stromal lamella. This explains the characteristic central corneal opacity (leukoma). Iridocorneal adhesions are frequently seen [Wenniger-Prick and Hennekam, 2002]. In the sever variety of this condition, the lens is adherent to the cornea. Isolated Peters anomaly is in general sporadic. Inherited forms are usually autosomal recessive but dominant forms also exist [Boel et al., 1979; Holmstrom et al., 1991]. Peters anomaly has been described in several chromosome anomalies [Goode-Jolly and Bonnin, 1966; Bateman et al., 1984; Cibis et al., 1985; Mayer, 1992; Temple et al., 1999]. Anterior segment defects of the eye are also found in many syndromes [Wenniger-Prick and Hennekam, 2002].
The term Peters-plus syndrome was first coined in 1984 by van Schooneveld et al. when they described 11 patients with a defect of the anterior segment of the eye, a typical face, clefting, short limb dwarfism, and developmental delay. The features of 49 cases with Peters-plus syndrome were summarized in 2002 by Wenniger-Prick and Hennekam. In addition to Peters anomaly these children have prenatal and postnatal growth retardation and are developmentally delayed. They have characteristic facial appearance which includes a round face in infancy, a long philtrum, and cupid bow's shape of the upper lip with a thin vermilion border. Cleft lip/palate can be present in 33–45% of cases. Short limbs and brachydactyly are invariably present. Other skeletal abnormalities include diminished mobility of the elbows, pectus excavatum, segmentation defects of the vertebral column and scoliosis [Wenniger-Prick and Hennekam, 2002]. Growth hormone deficiency responding to growth hormone replacement was found in one pair of sibs only [Wenniger-Prick and Hennekam, 2002].
Peters-plus syndrome is caused by mutations in the B 1, 3-galactosyltransferase-like gene (B3GALTL gene). All 20 patients with Peters-plus syndrome who were screened had homozygous or double heterozygous mutations in this gene [Lesnik Oberstein et al., 2006]. This finding placed Peters-plus syndrome in the list of congenital malformation syndromes caused by glycosylation defects.
The children in this report had many of the features described in Peters-plus syndrome including Peters anomaly of the eye, round face with cupid bow of the mouth in infancy, small hands and feet, intrauterine, and postnatal growth retardation. However, these children had in addition growth hormone deficiency and hypoplastic pituitary gland in one of them. Growth hormone deficiency in Peters-plus syndrome has been reported once only in one pair of sibs [Wenniger-Prick and Hennekam, 2002] and hypoplasia of the pituitary gland has not been reported previously in this syndrome. In addition, sequencing the whole B3GALTL gene did not reveal any mutation in these children making the diagnosis of Peters-plus syndrome unlikely.
Jung et al. 1995 described two children from two unrelated families with anterior chamber cleavage disorder, growth retardation, and dysmorphic features which were different from those found in Peters-plus syndrome. In addition these children had growth hormone deficiency, hypothyroidism, cerebellar abnormalities, and tracheal stenosis. Jung et al. 1995 suggested that the syndrome in these children could represent widening of the clinical spectrum of Peters-plus syndrome or it represent a distinct previously undescribed syndrome. However, the B3GALTL gene was not studied in this family. The children in this report have many of the features mentioned in this syndrome (Table I). These include anterior chamber anomalies of the eyes, growth retardation, round face with triangular mouth in infancy, small hands with spindle shaped fingers and endocrine abnormalities. The endocrine abnormalities in the children described by Jung et al. 1995 included growth hormone deficiency and hypothyroidism. However, no neuroimaging of the pituitary gland was done. In addition, there was cerebellar hypoplasia, and the possibility of corpus callosum hypoplasia in one of them. The children in this report had growth hormone deficiency, hypoplastic pituitary gland in one child, and hypoplastic corpus callosum in the other but normal thyroid gland function and normal cerebellum. It is possible that these differences are due to variable expression of the gene.
| Features | Case 1 | Case 2 | Peters plus |
Jung et al. 1995 |
|---|---|---|---|---|
| General | ||||
| Growth failure | + | + | + | + |
| Microcephaly | + | + | ± | + |
| Facial Features | ||||
| Round face as a child | + | + | + | |
| Telecanthus | − | − | + | − |
| Depressed nasal bridge | + | − | + | + |
| Broad Nose | + | + | + | + |
| Long philtrum | − | − | + | − |
| Cupid Bow | +in infancy | +in infancy | + | + |
| Neuroimaging | ||||
| Dilated ventricles | − | − | ± | − |
| Hypoplasia of CC | − | + | ± | ± |
| Cerebellar hypoplasia | − | − | ± | + |
| Absent/hypoplastic pituitary | + | + | − | − |
| Skeletal | ||||
| Short limbs | − | + | + | − |
| Short/small hands | + | + | + | ± |
| Broad hands | − | − | + | ± |
| Broad feet | − | − | ± | ± |
| Small feet | + | + | − | − |
| Vertebral anomaly | − | − | ± | ± |
| Dysplastic hip | − | − | ? | + |
| Ocular | ||||
| Peters anomaly | + | + | + | ± |
| Other anterior chamber | − | − | + | ± |
| Microphthalmia | − | − | − | + |
| Cataract | + | − | ± | + |
| Iris coloboma | − | − | − | ± |
| Glaucoma | − | − | + | + |
| Endocrine | ||||
| Growth Hormone deficiency | + | + | ±(one report) | + |
| Hypothyroidism | − | − | − | + |
| Others | ||||
| Tracheostenosis | − | − | − | + |
| Kidney abnormalities | − | − | ± | − |
| CHD | + | − | ± | − |
| Developmental delay | + | + | ± | + |
Other syndromes considered include the syndrome described by Moog et al. 1998 in which two sibs had anterior chamber defect, growth retardation, hydrocephaly with intracranial calcifications, and mild mental retardation. However, there was no hydrocephaly or intracranial calcification in the sibs in this report. Similarly anterior segment anomalies of the eyes with growth retardation are features of Al-Gazali syndrome [Al-Gazali et al., 1999] however the lack of skeletal abnormalities and different facial appearance ruled this diagnosis out. Patients manifesting both hypopituitarism and ophthalmological defects have been reported with chromosomal deletion of 14q22-q23 [Bennett et al., 1991; Elliott et al., 1993; Lemyre et al., 1998]. Mutation in the BMP4 was found in some of these patients [Bakarnia et al., 2008]. However, the ocular abnormalities and the facial appearance in our patients are different from these cases and a detailed chromosome analysis was normal in our patients. Ragge et al. 2005 described eight unrelated families with unilateral or bilateral microphthalmia and variable additional features including coloboma, microcornea, cataract with hypoplasia or agenesis of the optic nerve, agenesis of the corpus callosum, developmental delay, joint laxity with hypotonia, and seizures. The authors identified heterozygous mutations in the OTX2 gene located on chromosome 14q21-q22 in these patients. There was no hypopituitarism, the ocular abnormalities and the facial features are different from the cases in this report. Another entity which combines ocular anomalies with hypopituitarism is the Oculo-pituitary syndrome [London Medical Data Base, 2007]. The ocular anomalies include colobomas, amyblyopia, microphthalmia, and optic nerve aplasia but not anterior segment anomalies.
It is possible therefore, that the children in this report have a previously not described syndrome. On the other hand, they could represent variable manifestation of Jung syndrome. Due to the recurrent association of ocular anomalies and pituitary dysfunction it might be reasonable also to recommend endocrine assessment for all children with anterior segment anomalies of the eye.
Appendix 1: Pedigree
Peripheral blood samples were taken from family members after informed consent forms were signed. Genomic DNA was isolated from the white blood cells following standard established protocols. The primers to amplify the 15 coding exons of the B3GALTL gene with the flanking intronic sequences including the splice sites sequences were designed using Primer3 software available online (shown bellow) and custom made by Metabion Inc., Germany (www.metabion.com). Each pair of primers were used in a standard 30 µL PCR reactions consisting 1X PCR buffer (Qiagen), 1X Q-buffer (Qiagen), 1 µM of each of the forward and reverse primers 0.2 mM of each of the dNTPs, ∼50 ng of genomic DNA and 0.15 µL of Qiagen Taq DNA polymerase (5 units/µL). PCR was carried out according to the following protocol: (94°C for 5 min, 1 cycle; 94°C for 30 sec, 57°C for 1 min, 72°C for 1 min, 35 cycles; 72°C for 10 min). PCR reactions products were initially analyzed using 1.8% Agarose gel electrophoresis and 5 µL of the successful reactions were treated for 30 minutes at 37°C with 1 µL Exo-SAP-IT (Roche) followed by inactivation of the enzymes at 80°C for 20 min. This was performed to clean of the PCR products from excess primers and dNTPs. DNA sequencing was performed using the ABI 3730 × l gene analyzer at Macrogen Inc., South Korea (www.macrogen.com). The obtained sequences were compared to that reported on the human genome browser for B3GALTL gene (uc001utm.1).
Appendix 2: DNA Analysis of B3GALTL Gene
-
Bgalex1-F cgcagaggagaaaggaagag
-
Bgalex1-R ggacccaagaccgaaagg
-
Bgalex2-F cagctgcttaaaaatgagcaaa
-
Bgalex2-R aaccaagtggatcagccttaaa
-
Bgalex3-F tacatcattgctccgtggtc
-
Bgalex3-R caggagagcaaacgttaggc
-
Bgalex4-F tgcctgaaggtttgctttgt
-
Bgalex4-R ccagctgaccgaaacatttt
-
Bgalex5-F1 tgcattttaagccaagccttt
-
Bgalex5-R1 tgaggaaaaccacaccctctt
-
Bgalex6-F gccattctgtgtacccttcatt
-
Bgalex6-R agtttccaactctctgaaattgct
-
Bgalex7-F tctgaaaccaatagtaccaccttcta
-
Bgalex7-R caagcatctggctccttttc
-
Bgalex8-F1 agccgttgggttttcacagt
-
Bgalex8-R1 cacctaattcgctggagtgc
-
Bgalex9-F tggttcagcctactctctatgga
-
Bgalex9-R aagcccaaaccttagcatca
-
Bgalex10-F tgtttggtatccttgacattgg
-
Bgalex10-R tccgaattgtcattatcccttt
-
Bgalex11-F ggaagcaagcaactcttgga
-
Bgalex11-R caatcagtatctgagagaagggaaa
-
Bgalex12-F ttaagctgcattttggcatg
-
Bgalex12-R cgtccccgttacttcatctt
-
Bgalex13-F cagagtgggatgtaagaaccataa
-
Bgalex13-R tcccagtgccagagacctac
-
Bgalex14-F gcctctcagtctgcaggagt
-
Bgalex14-R tggagcctgtcaacacagtt
-
Bgalex15-F tgcttttagattcctatagccaatg
-
Bgalex15-R tgaccccttttcttcctctg