Further clinical delineation of the Börjeson–Forssman–Lehmann syndrome in patients with PHF6 mutations†
How to cite this article: Carter MT, Picketts DJ, Hunter AG, Graham GE. 2009. Further clinical delineation of the Börjeson–Forssman–Lehmann syndrome in patients with PHF6 mutations. Am J Med Genet Part A 149A:246–250.
Abstract
Börjeson–Forssman–Lehmann syndrome is an X-linked condition caused by PHF6 mutations. The classical description of males with this disorder includes severe intellectual disability with epilepsy, microcephaly, short stature, obesity, hypogonadism, and gynecomastia. We present three males with PHF6 mutations whose features included deep-set eyes, large ears, coarse face, tapering fingers, and truncal obesity. Unlike the original description of the syndrome; however, the males described herein had varying degrees of intellectual disability and hypogonadism, were of normal to tall stature, had normal to large head sizes, and did not have seizures. This departure from the usual clinical description of Börjeson–Forssman–Lehmann syndrome is consistent with recent reports of males with mutations in PHF6. In addition, we describe the phenotype and X-inactivation pattern in two females heterozygous for PHF6 mutations, both of whom have mild features of the syndrome. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Börjeson–Forssman–Lehmann syndrome (BFLS; OMIM 301900) is an X-linked condition that causes intellectual disability, distinctive facial features, truncal obesity, and gynecomastia in affected males. It was first reported in three related males with severe mental retardation, epilepsy, short stature, and microcephaly [Börjeson et al., 1962]. The locus for BFLS subsequently was mapped to the X-chromosome [Mathews et al., 1989; Turner et al., 1989], and mutations in the PHF6 gene were shown to cause the disorder [Lower et al., 2002]. Mutations in PHF6 have been reported in 13 families and six sporadic cases [Lower et al., 2004; Turner et al., 2004; Vallée et al., 2004; Visootsak et al., 2004; Crawford et al., 2006].
The phenotypic spectrum of BFLS in males has proven to be more variable than reported in the original family. The degree of intellectual impairment ranges from mild to severe, and epilepsy is an inconsistent, even uncommon, feature [Turner et al., 2004; Gecz et al., 2006]. Although the males in the original family were microcephalic, it is now clear that head size can range from microcephalic to macrocephalic [Turner et al., 2004]. Features that are consistent among males with mutation-proven BFLS include deep-set eyes, large ears, a coarse face, tapering fingers, short toes, gynecomastia, hypogonadism, truncal obesity, and hypotonia [Turner et al., 2004; Gecz et al., 2006].
Females heterozygous for PHF6 mutations have phenotypes that range from unaffected to full clinical manifestations identical to those reported in male patients [Lower et al., 2002; Turner et al., 2004]. Skewing of X-chromosome inactivation has been postulated as the mechanism of this variability [Kubota et al., 1999; Turner et al., 2004]. However, the pattern of X-inactivation in peripheral blood from female heterozygotes has not always been predictive of the phenotype [Crawford et al., 2006].
We describe three male patients with mutation-proven BFLS, and two female heterozygotes. The mutation analyses for the individuals described herein were published previously [Vallée et al., 2004]. In this report, we provide detailed clinical information to further delineate the phenotypic spectrum of BFLS in both males and females. We highlight the variable phenotype in males with BFLS and some signs not described previously. We also report on the X-inactivation pattern in a mildly affected mother and daughter both heterozygous for the PHF6 mutation.
CLINICAL REPORTS
Patient 1 (Fig. 1) was the product of an unremarkable pregnancy in which there were no maternal illnesses or reported teratogenic exposures. His parents were unrelated Caucasians. He was born following an uncomplicated spontaneous vaginal delivery with a birth weight of 3.6 kg (50–75th centile). He had an early history of hypotonia, poor coordination, and global developmental delay, with particular difficulty in the acquisition of speech. The quality of his speech was improved by surgery for ankyloglossia at 3 years. Recurrent otitis media required bilateral tube insertions at 5 years. He had a right orchidopexy for cryptorchidism at age 11 years. At age 15 years, spine X-ray showed prominent thoracic kyphosis and lumbar lordosis.
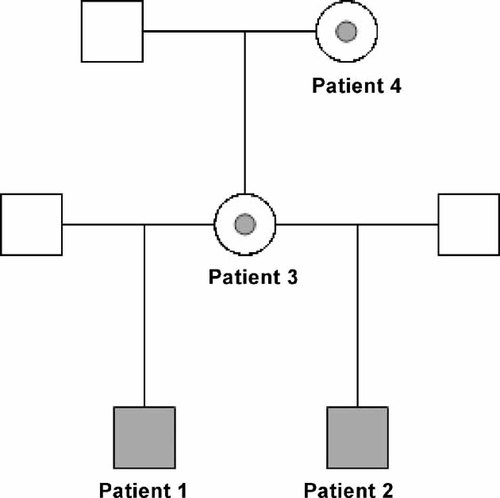
Pedigree showing relationships of Patients 1–4. Filled squares indicate males with a hemizygous PHF6 mutation, and circles with gray dots indicate females heterozygous for the PHF6 mutation.
Formal psychometric testing at 4.5 years demonstrated that he was functioning at a 2.5-year level; at 7.5 years, his delay was 3 to 3.5 years. He was diagnosed with attention deficit hyperactivity disorder (ADHD), which was treated with methylphenidate and subsequently dextroamphetamine. At 14 years and 7 months, he was in grade 9 in a special education class, performing at a grade 2 level. His behavior is an ongoing problem, and he has been admitted to a psychiatric ward for intensive management of obsessive-compulsive disorder, ADHD, and aggressive behavior. He was taking daily medications including risperidone, methylphenidate, clonidine, and trazadone.
The diagnosis of Börjeson–Forssman–Lehmann syndrome was made based on clinical features at age 7 years 11 months. His OFC was 50.8 cm (10–25th centile), height 124.1 cm (25th centile), weight 27.5 kg (75th centile), and BMI 17.9 kg/m2 (75–90th centile). He had a right frontal upsweep, heavy brows, epicanthus inversus, deep-set eyes, short nose with a square tip and upturned, narrow base; a strong chin, and full cheeks (Fig. 2A). His ears were both 6.5 cm long (+2 SD). He had tapering digits with relative shortness of the index and fifth fingers and generalized hyperextensibility of the joints. His toes appeared short (Fig. 2A). He had an accessory nipple on the left abdomen. At 14 years 7 months, his OFC was 54.5 cm (50th centile), height 165.1 cm (just below 50th centile), and he weighed 86 kg (+3 SD) with a BMI of 31.6 kg/m2 (+3 SD). He had truncal obesity with bilateral breast enlargement, which on palpation was mostly adipose tissue. Acanthosis nigricans was present on his posterior neck and axillae. Glycosylated hemoglobin A1C was 0.058 (normal range of 0.04–0.06) and fasting insulin level was 118 pmol/L (normal range of 13–161 pmol/L). He entered puberty spontaneously at 13 years of age. His penis was small and the testes were of 8–10 mL but firm. Luteinizing hormone (LH) level was 4 IU/L (normal range of 1–9 IU/L) and follicle stimulating hormone (FSH) level was 4 IU/L (normal range of 1–19 IU/L).
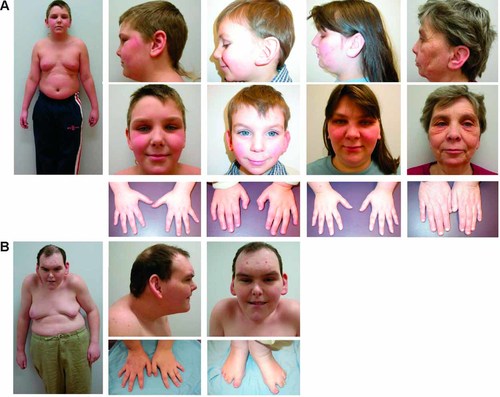
(A) Left to right: Patient 1, 9 years 11 months. Note central obesity, breast enlargement, large ears, deep-set eyes, and tapering fingers. Patient 2 (the proband), 2 years 5 months. Note large, posteriorly rotated ears, and relatively non-dysmorphic appearance. Patient 3, mother of Patients 1 and 2. Note fleshy, posteriorly rotated ears, heavy brows, and tapering fingers. Patient 4, mother of Patient 3. Note deep-set eyes, fleshy, posteriorly rotated ears, and small nails. (B) Patient 5, 27 years. Note stooped posture, truncal obesity, breast enlargement, frontal balding. Face is triangular and coarse. Ears are large and fleshy. Eyes are deep-set and palpebral fissures are narrow. Fingers are tapered and toes are short with almost complete cutaneous 2–3 syndactyly bilaterally. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Sequencing of the PHF6 gene revealed ac.139-8A>G mutation, which predicts a cryptic acceptor splice site and enhanced exon skipping [Vallée et al., 2004].
Patient 2 (Fig. 1) was referred to the Genetics clinic at 11 months of age for developmental delay. He was the product of an unremarkable pregnancy in which there were no maternal illnesses or reported teratogenic exposures. He was born following an uncomplicated vaginal vertex delivery at 41 weeks. Birth weight was 3.8 kg (75th centile). His medical history included mild neonatal jaundice that did not require treatment, otitis media, and asthma. He had a history of poor feeding and failure to thrive around 6 months of age. He was followed by Child Development services from ages 2 to 5 years, and noted to be delayed in all areas of development, with the most difficulty in language acquisition. At 3 years old he was formally assessed by a speech pathologist who deemed him to be 1 year behind in his language skills. When he entered school he was placed in a special class for learning disabled children.
At 2 years 5 months, his OFC was 47.3 cm (<3rd centile), height 86.9 cm (25th centile), and weight 11.2 kg (3rd–10th centile). His BMI was 14.8 (−2.5 SD). He had prominent and posteriorly rotated ears measuring 6.3 cm bilaterally (+3 SD), a right preauricular pit and short nails. His forehead was high and his hair was sparse in the temporal areas. He had upslanting palpebral fissures and deep-set eyes (Fig. 2A). The philtrum was flat with a thin, down-turned upper lip. He had small, widely spaced teeth and pointed canines. There was no truncal obesity or gynecomastia. His fingers were only mildly tapered, but the terminal phalanges of the index fingers appeared short and the nails small. At 8 years of age, his OFC was 50.9 cm (25th centile), height 124.6 cm (50th centile), weight 27.6 kg (75th centile), and BMI was 17.8 (75–90th centile). He was beginning to accumulate central adiposity. There was no breast enlargement. His testicular volume was 1–2 ml. He was on daily medications including risperidone and methylphenidate. His guardians reported increasingly aggressive behavior, mood changes and anxiety; his psychiatrist has diagnosed him with ADHD and reactive attachment disorder.
Sequencing of the PHF6 gene revealed the same c.139-8A>G mutation as found in his brother.
Patient 3 (Fig. 1), the mother of Patients 1 and 2, was born at term. Further birth history is not available. She had no significant medical problems. Developmental milestones were appropriate. She repeated grade one because she had trouble with reading, but thereafter, although she performed poorly in school, she passed her classes. A learning disability was confirmed with formal psychometric testing (WISC-R full-scale score in the borderline to low-average range) at 15 years of age. She has struggled long-term with anxiety and depression, which have been treated on an outpatient basis since early adolescence. At 27 years of age, her OFC was 56 cm (75th centile), height 167 cm (75th centile), weight 103.8 kg (+4SD), and BMI 37.2. She had heavy eyebrows, a mild degree of synophrys, deep-set eyes, narrow-appearing palpebral fissures, a poorly grooved philtrum, and a thin upper lip. Her ears were posteriorly rotated and measured 7.7 cm bilaterally (>98th centile) with fleshy lobes. Her fingers were tapering and hyperextensible (Fig. 2A).
This woman had the same heterozygous c.139-8A>G mutation in the PHF6 gene as her sons. X-inactivation testing, performed by methylation analysis of the human androgen receptor gene [Allen et al., 1992], revealed non-significant skewing with 38% inactivation of the mutation-bearing X chromosome.
Patient 4 (Fig. 1), the maternal grandmother of Patients 1 and 2, has a history of learning disability (not formally diagnosed) and repeated two grades in school. Further, birth and medical history is not available. At 56 years of age, her OFC was 55.6 cm (just >50th centile), height was 157.5 cm (25th centile), weight was 60 kg, and BMI was 24.2. She had a right-sided frontal upsweep, heavy eyebrows, and deep-set eyes. The philtrum was long and poorly grooved, with a thin and downturned upper lip (Fig. 2A). She had large, posteriorly rotated ears measuring 7.1 cm bilaterally (>98th centile) with fleshy lobes. She did not have central obesity. Her fingers were not tapering; however, the nails were small. Her toes appeared short and her toenails were deep-set and narrow (Fig. 2A).
This woman had a heterozygous c.139-8A>G mutation PHF6. X-inactivation studies revealed 89% inactivation of the mutation-bearing X-chromosome.
Patient 5 is unrelated to Patients 1–4. He was born at 41 weeks gestation, weighing 3.38 kg. The pregnancy and delivery were uncomplicated. His past medical history includes severe global developmental delay, ankyloglossia requiring surgery, strabismus, unilateral inguinal hernia, bilateral cryptorchidism, and hypospadias. He was prescribed glasses for astigmatism in childhood. Between the ages of 10 and 12 years he developed obesity and enlarged breasts. At 16 years he was diagnosed with Hodgkins lymphoma and was treated with radiation. At 22 years he demonstrated increasingly aggressive behavior that required a 7-month psychiatric hospitalization. He was treated for an anxiety disorder and mood disturbance. Both were well managed with medications, although he remained self-injurious. His past investigations include a normal chromosome analysis and cytogenetic test for fragile X syndrome. At 27 years of age, his OFC was 58.1 cm (98th centile), height was 168.3 cm (just >10th centile), his weight was 91 kg and his BMI was 32.1. He had a markedly stooped and kyphoscoliotic posture with narrow, rounded shoulders, truncal obesity, and bilateral gynecomastia. He had no facial, axillary, or chest hair. He had frontal balding and excoriations over the forehead from self-injury. The face was triangular and coarse with heavy eyebrows, synophrys, prominent supraorbital ridges, and deep-set eyes with narrow palpebral fissures. There was bitemporal narrowing with prominent zygomata, a narrow nasal root and bridge, short nose with a triangular upturned base. The philtrum was smooth with a thin upper lip and downturned mouth (Fig. 2B). He had poor oral hygiene, necessitating multiple dental extractions. The patient was also prognathic and had large ears measuring 8 cm bilaterally (>98th centile) with fleshy lobes. He had tapering and hyperextensible fingers with small nails, short toes with small nails, a wide sandal gap and almost complete 2–3 cutaneous toe syndactyly (Fig. 2B). His testes and penis were small, with Tanner stage 2 or 3 pubic hair. Free testosterone and DHEAS were low at 19.1 pmol/L and 2.6 umol/L, respectively. His LH level was 7 IU/L (normal range of 1–9 IU/L) and FSH level was 8 IU/L (normal range of 1–19 IU/L).
Sequencing of the PHF6 gene revealed a hemizygous variant c.769A>G (which predicts p.R257G) in exon 8 [Vallée et al., 2004].
DISCUSSION
Before the identification of PHF6 as the gene responsible for BFLS, the diagnosis was based on clinical features described in the original report, which included severe intellectual disability, epilepsy, short stature, hypogonadism, obesity, narrow palpebral fissures, and large ears [Börjeson et al., 1962]. Publication of clinical descriptions of mutation-positive individuals allowed for further delineation of the clinical features of BFLS. To date, over 40 such individuals have been described [Turner et al., 1989; Baumstark et al., 2003; Lower et al., 2004; Turner et al., 2004; Crawford et al., 2006]. Those described in detail in the literature are summarized in Table I. Mutation-positive males have intellectual disability, obesity, gynecomastia, and small external genitalia. Other common descriptions include a characteristic “heavy” or “coarse” face with deep-set eyes and large, fleshy ears, tapering fingers, and broad feet with short and/or flexed toes [Turner et al., 2004; Gecz et al., 2006].
| Clinical Finding | Malesa | Femalesab |
|---|---|---|
| Intellectual disability | 43/43 (100%) | 5/21 (24%) |
| Obesity | 20/26 (77%) | 3/4 (75%) |
| Gynecomastia | 38/39 (97%) | n/a |
| Microcephaly | 2/31 (6%) | 0/3 (0%) |
| Macrocephaly | 4/31 (13%) | 1/3 (33%) |
| Seizures | 3/39 (8%) | 1/17 (6%) |
| Short stature | 13/37 (35%) | 0/3 (0%) |
| Small penis and/or testes | 14/18 (78%) | n/a |
- n/a, not applicable.
- a Total represents the number of patients on whom appropriate information is available.
- b Includes one female proband and females ascertained through their affected son(s).
The facial gestalt of our patients is similar to those reported in Turner et al. [2004], in which they show males with BFLS at different ages, ranging from infancy to adulthood. The younger males (Patients 1 and 2) have a less distinctive facial gestalt than the adult male (Patient 5), consistent with the observations of Turner et al. [2004] that the characteristic face becomes apparent in late childhood, concomitant with the onset of obesity. It is of note that the diagnosis in our family was first suspected (by AH) in the maternal grandmother who brought patient 2 to a clinic appointment; the boy's developmental delay was not initially attributed to BFLS. Unlike the originally reported family with BFLS, which subsequently was shown to carry a mutation in PHF6 [Lower et al., 2002], our patients are of average stature, with head sizes varying from microcephalic (Patient 2) to normocephalic (Patient 1) to macrocephalic (Patient 5). Seizures were included in the original description of BFLS, but are not present in our patients. The literature also suggests that seizures are uncommon in BFLS (Table I). Enlarged breasts are reported as “gynecomastia” in the literature, but it is unclear whether this is caused by breast tissue hyperplasia or lipomastia. Patients 1 and 5 both had enlarged breasts, but palpable breast tissue was present only in Patient 5. Aggressive behavior has not previously been reported in BLFS males, but all three of our patients required therapy for this; further study is needed to determine if this is a consistent aspect of the behavioral phenotype of BFLS. Our patients also had a few other signs not previously reported, including ankyloglossia (Patients 1 and 5) and accessory nipple (Patient 1). It is not clear whether the diagnosis of Hodgkin's lymphoma in Patient 5 is coincidental or a rare finding in BFLS.
Clinical data on the phenotype in females heterozygous for PHF6 mutations is sparse. Most are ascertained through their affected male relatives. Descriptions vary from intellectually normal without physical features of BFLS, to significant intellectual disability and/or physical features consistent with BFLS [Turner et al., 1989; Lower et al., 2004; Turner et al., 2004] (Table I). The degree of skewing of X-inactivation, which is a relatively common feature of some X-linked mental retardation disorders [Plenge et al., 2002], is not correlated with the BFLS phenotype [Baumstark et al., 2003; Crawford et al., 2006], and our data support this. The X-inactivation patterns of our two affected female patients were dissimilar, although both had features consistent with BFLS.
We report on the clinical features of three males with mutation-proven BFLS, and two heterozygous females with mild clinical features. A consistent physical phenotype is emerging, but is more variable than the original description, especially for head size and height. Women with a PHF6 mutation may show clinical features of BFLS, but the pattern of X-chromosome inactivation does not always correlate with phenotype. The diagnosis of BFLS must be considered in the context of the family, as the phenotype in young boys may not be readily apparent, but the mother may show subtle signs of the classical clinical description.
Acknowledgements
The authors thank the patients and their families for their kind participation, including their permission to publish their photographs.




