A novel CDKL5 mutation in a 47,XXY boy with the early-onset seizure variant of Rett syndrome†‡
Stefano Sartori and Gabriella Di Rosa contributed equally to this work.
How to cite this article: Sartori S, Di Rosa G, Polli R, Bettella E, Tricomi G, Tortorella G, Murgia A. 2009. A novel CDKL5 mutation in a 47,XXY boy with the early-onset seizure variant of Rett syndrome. Am J Med Genet Part A 149A:232–236.
Abstract
Mutations of the cyclin-dependent kinase-like 5 gene (CDKL5), reported almost exclusively in female subjects, have been recently found to be the cause of a phenotype overlapping Rett syndrome with early-onset epileptic encephalopathy. We describe the first CDKL5 mutation detected in a male individual with 47,XXY karyotype. This previously unreported, de novo, mutation truncates the large CDKL5 COOH-terminal region, thought to be crucial for the proper sub-cellular localization of the CDKL5 protein. The resulting phenotype is characterized by a severe early-onset epileptic encephalopathy, global developmental delay, and profound intellectual and motor impairment with features reminiscent of Rett syndrome. In light of the data presented we discuss the possible phenotypic modulatory effects of the supernumerary wild type X allele and pattern of X chromosome inactivation and stress the importance of considering the causal involvement of CDKL5 in developmentally delayed males with early-onset seizures. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5) gene have recently been found in patients with severe neurodevelopmental disorders, including infantile epileptic encephalophathy, severe X-linked infantile spasms (ISSX), and mental retardation, autism, and the early-onset seizure variant of Rett syndrome, also known as the Hanefeld variant [Tao et al., 2004; Weaving et al., 2004; Scala et al., 2005]. Although the biological function of the CDKL5 protein has not yet been clearly elucidated, it appears to be functionally related to the methyl CpG-binding protein 2 (MECP2), the gene of which is mutated in most classical cases of Rett syndrome (RTT).
Here we report on a de novo, novel, CDKL5 gene mutation found for the first time in a boy with early-onset epileptic encephalopathy, and a 47,XXY chromosomal constitution.
CLINICAL REPORT
We describe a 4-year-old boy, first child of non-consanguineous parents, born at term after an unremarkable pregnancy and delivery. At birth the weight of the child was 2.9 kg (10–25th centile), the length 49 cm (10–25th centile) and the occipitofrontal circumference 33 cm (10–25th centile). The immediate perinatal period was reported as uneventful.
At 15 days of life the child begun to present sporadic extensor and flexor spasms associated with eyelid myoclonia and oral automatisms followed by tonic hyperextension of the four limbs. With time, seizure frequency increased significantly (5–10 clusters, each with 10–15 spasms, per day). The child was brought to our attention for the first time at 3 months of age. The first ictal EEG showed high amplitude central bilateral spikes during the spasms, whereas the interictal EEG was normal. The clinical examination showed acquired microcephaly (38 cm, 3rd centile), and subtle facial anomalies (telecantus, upslanting eyelids, thin lips). Mild generalized hypotonia and head lag, with reduced eye contact were also evidenced. Treatment with valproic acid was initiated with poor results: the child continued to manifest seizures which rate increased throughout the first year of life. Starting from 6 months of age, the child manifested myoclonic and generalized tonic–clonic seizures, and the EEG begun to display a progressive slowing of the background activity.
At the age of 1-year a severe psychomotor delay and generalized hypotonia were evident and the child still manifested severe epilepsy, despite several attempts of pharmacologic treatment with topiramate, lamotrigine, levetiracetam, clonazepam, clobazam, hydrocortisone, ethosuximide, phenobarbital, and ACTH.
When the child was about 2 years old, he started to present dystonic-athetoid movements and apnea and breath-holding episodes without EEG correlates. At this age the child was unable to sit alone; speech and communicative abilities were virtually absent. Moreover, autism-like features as absence of eye contact and stereotyped hand movements (e.g., hand-wringing or washing) were evident. The body length was beyond the 97th centile, whereas weight and occipitofrontal head circumference were both under the 3rd centile. The frequency of seizures was essentially unchanged: spasms followed by a tonic phase, myoclonic, and generalized tonic–clonic seizures persisted. The interictal EEG, obtained during wake and sleep, disclosed an electrical pattern consisting in pseudoperiodic, diffuse, high-amplitude sharp waves, spikes and polyspikes mainly localized in the pre-central and central regions.
For the subsequent years the child did not show any developmental gain. Now, at the age of 5 years the patient shows some mildly dysmorphic facial features, resembling aspects of both parents (Fig. 1). He has profound intellectual impairment associated with marked hypotonia and absence of postural control; his seizures are unvaried and very difficult to control. Several investigations, performed in the course of the years, were consistently negative: routine laboratory work-up, metabolic tests, and multi-modal evoked potentials (VEP, ABR, SSEP, EMG, and neurography). Brain MRI performed at 11 and 17 months showed moderate atrophy of the frontal lobes and mild T2-weighted periventricular hyperintensities.
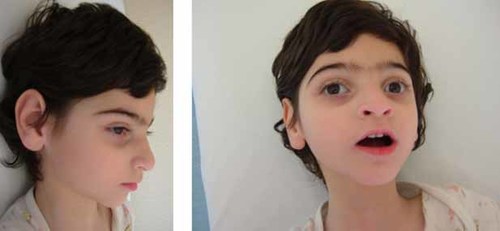
The patient at 5 years of age, showing the following features: synophrys, large ears, thin upper lip, pointy chin. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
MATERIALS AND METHODS
Cytogenetic Analysis
A CTG banding standard karyotype was performed on peripheral blood cultures.
Molecular Analysis
Regular informed consent for genetic testing was obtained from the child's parents. Genomic DNA was extracted from peripheral blood leukocytes (PBL) with standard methods. Determination of the parental origin of the extra X-chromosome was obtained with the study of the androgen receptor and FMR1 genes polymorphisms.
Mutation screening of the MECP2 and CDKL5 genes was performed by PCR amplification and subsequent scanning of the entire coding sequence, and intron–exon boundaries, with the use of Transgenomic Wave denaturing high-performance liquid chromatography (dHPLC). Primer sequences and amplification conditions as previously described [Amir et al., 1999; Kalscheuer et al., 2003; Mnatzakanian et al., 2004].
Melting profiles and dHPLC running conditions were determined with the use of the Navigator 1.7.0 software. PCR products with abnormal dHPLC profiles were sequenced on ABI 3100 genetic analyzer (PE Applied Biosystems, Foster City, CA).
Amplification products of exons 1 and 16 of the CDKL5 gene were directly sequenced due to the known frequency of polymorphic variants in these exons.
A quantitative real-time PCR of the MECP2 coding sequence was performed, according to a previously described method [Casarin et al., 2006], on two fragments comprised between nucleotides 904–1,033 and nucleotides 1,311–1,474 for detection of dose alterations due to large deletions or duplications.
X-Chromosome Inactivation
Patterns of X-inactivation were determined in peripheral blood leucocytes by polymerase chain reaction analysis of the polymorphic CAG repeat in the first exon of the androgen receptor (AR) gene after digestion with methylation-sensitive restriction enzyme HpaII (New England Biolabs, Ipswich, MA) [Allen et al., 1992].
The AR locus was PCR amplified from digested and undigested DNA using fluorochrome–labeled primers. Allele peak areas were analyzed using an ABI 3100 automated sequencer and the GeneScan software (PE Applied Biosystem).
The degree of X-inactivation skewing was calculated as the fractional peak–height ratio (expressed as %) for the more strongly amplified allele; X-inactivation was considered significantly skewed if the ratio exceeded 75:25. DNA samples from the parents were genotyped to determine the parental origin of the alleles.
RESULTS
The cytogenetic analysis revealed the presence of two copies of the X-chromosome (47,XXY by ISCN 2005).
The study of the two X-chromosome polymorphic regions, FMR1 CGG, and androgen receptor CAG repeats, on the DNA of the patient and of both parents, allowed to establish the paternal origin of the extra copy of the X-chromosome, indicating a non-disjunction event at paternal first meiotic division.
A mutation scanning of the MECP2 gene did not detect point mutations or small insertion/deletions. The quantitative PCR analysis, performed on two different fragments within exon 4, evidenced a double dose of MECP2 genomic DNA, consistent with the results of the karyotype.
Molecular analysis of the CDKL5 gene revealed the presence of an altered dHPLC profile of the amplicon corresponding to exon 12. Direct sequencing of the altered fragment allowed the identification of a heterozygous single base substitution at nucleotide 1,675 of the coding sequence (c.1675C > T: Ref. Seq. NM_001037343) resulting in the substitution of the Arginin residue at position 559 of the amino-acid sequence with a stop codon (p.Arg559Stop) (Fig. 2). The mutation was not present in the DNA of the parents. A balanced pattern of X-chromosome inactivation (50% and 50%) was found in the patient's DNA sample.
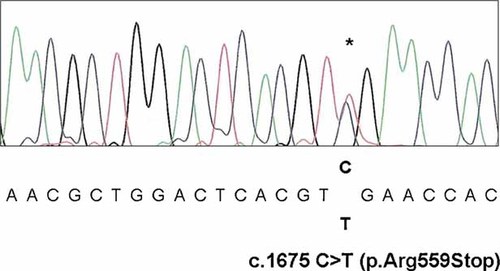
Direct sequencing of the CDKL5 amplicon harboring a heterozygous C to T substitution, c.1675C > T (asterisk), resulting in the substitution of the Arginin residue at position 559 of the aminoacid sequence with a stop codon (p.Arg559Stop). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
RTT is a severe progressive neurodevelopmental disorder that primarily affects females. Classic RTT girls show, after a seemingly normal early development, loss of acquired motor and speech skills, followed by the appearance of stereotypic hand movements, autistic features, breathing disturbances, motor impairment, and seizures [Weaving et al., 2004; Williamson and Christodoulou, 2006; Chahrour and Zoghbi, 2007]. Several RTT variants, also called atypical RTT, have been described, including the “preserved speech variant,” the “forme fruste,” the “late regression variant,” the “congenital variant” [Scala et al., 2005; Sampieri et al., 2007], and the “early-onset seizures variant.” This latter, initially described in 1985 by Hanefeld, in female patients with atypical RTT and infantile spasms with hypsarrhythmia, has been subsequently extended to include atypical Rett patients with early-onset seizures [Goutières and Aicardi, 1985; Hanefeld, 1985].
Mutations of the MECP2 gene, found in 80–95% of classic RTT and in only 20–50% of the atypical forms [Buyse et al., 2000; Cheadle et al., 2000; Bourdon et al., 2001; Weaving et al., 2005], have never been reported in individuals with the Hanefeld variant [Evans et al., 2005; Scala et al., 2005].
Interestingly, some authors have recently reported that a subset of patients with clinical picture resembling the early-onset seizures variant of RTT carry mutations of CDKL5, a gene involved in a wide range of phenotypes, including infantile epileptic encephalophathy, severe X-linked infantile spasms (ISSX) and mental retardation, and autism [Tao et al., 2004].
The CDKL5 mutations so far reported have been detected almost exclusively in female patients, similarly to what happens for MECP2. Based on the lack of evidence of a high rate of miscarriage or gender skewing in the familial cases [Murphy et al., 1986], the female prevalence among individuals carrying MECP2 mutations has been hypothesized to be due to very early male lethality, preventing the recognition of pregnancies [Schanen, 2001]. Furthermore, sporadic MECP2 mutations tend to occur in male germ cells, males therefore would not inherit them. The same mechanisms might be suggested for CDKL5.
To date, four males have been reported with alterations involving the CDKL5 gene [Huopaniemi et al., 2000; Weaving et al., 2004; Van Esch et al., 2007]. Only in one of these cases however the mutation, a single base deletion at nucleotide 183 of the coding sequence, affects exclusively this gene which is therefore, by itself, fully responsible of a phenotype characterized by early-onset epileptic encephalopathy, severe global developmental delay, profound intellectual, and motor impairment [Weaving et al., 2004]. The brothers with X-linked juvenile retinoschisis (XLRS) reported by Huopaniemi et al. 2000 carried a 136-kb deletion, causing the loss of XLRS and PPEF-1 genes and an apparently ineffective C-terminal deletion of CDKL5; one of these children did not have neurological disturbances and in the other the epilepsy was likely due to neonatal hypoxic–ischemic brain injury. The patient reported by Van Esch et al. 2007 presented with a large deletion of Xp22, involving more than one gene.
Our case is therefore the second actual CDKL5 mutation in a male, the first one associated with a 47,XXY karyotype. The mutation detected interrupts the long COOH-terminal region of the CDKL5 gene introducing a stop codon between residues 526 and 780, crucial for the sub-cellular localization of the CDKL5 protein within the nucleus [Bertani et al., 2006]. This alteration is to be considered disease-causing and responsible of the severe early epileptic encephalopaty of our patient who does not present neurological and epileptological features that may be seen in Klinefelter individuals [Tatum et al., 1998; Ross et al., 2008].
Considering what reported in males with a 47,XXY karyotype or with MECP2 somatic mosaicism [Schwartzman et al., 2001; Vorsanova et al., 2001; Hammer et al., 2003; Chahrour and Zoghbi, 2007], a less severe phenotype would be expected in our patient, in comparison with the only other male carrying a mutation within the coding sequence of CDKL5 and 47,XXY karyotype [Weaving et al., 2004]. This could be hypothesized based on the possible favorable role played by the two X-chromosomes with a balanced pattern of inactivation. A relatively milder phenotype would also be justified, in our patient, by the presence of a C-terminal truncating mutation, which spares the NH2-terminal kinase domain of the CDKL5 protein.
Despite the speculations the phenotype of the two patients is not significantly different, even though comparable only up to the fifth year of life. The residual CDKL5 function, due to the double-dose of the gene and to the balanced pattern of X inactivation, does not seem to have a beneficial impact on the severity of the clinical condition in our 47,XXY patient.
The apparent discordance between the possible ameliorating effect conferred by the supernumerary wild type X-chromosome [Hammer et al., 2003; Tao et al., 2004] and the particularly severe phenotype observed could be explained either by a heavily detrimental function of the disease-causing mutation or by a discordant ratio of X inactivation in different tissues, which could theoretically be in favor of the mutated allele in the brain [Tan et al., 1993; Gale et al., 1994].
Finally, we would like to stress the importance of considering the causal involvement of CDKL5 even in males showing early onset seizures and Rett-like clinical features, as well as other phenotypes that have been related to mutations of this gene in females.




