Autosomal dominant inheritance of cardiac valves anomalies in two families: Extended spectrum of left-ventricular outflow tract obstruction†
How to cite this article: Wessels MW, van de Laar IMBH, Roos-Hesselink J, Strikwerda S, Majoor-Krakauer DF, de Vries BBA, Kerstjens-Frederikse WS, Vos YJ, de Graaf BM, Bertoli-Avella AM, Willems PJ. 2009. Autosomal dominant inheritance of cardiac valves anomalies in two families: Extended spectrum of left-ventricular outflow tract obstruction. Am J Med Genet Part A 149A:216–225.
Abstract
Only a limited number of families with clear monogenic inheritance of nonsyndromic forms of congenital valve defects have been described. We describe two multiplex pedigrees with a similar nonsyndromic form of heart valve anomalies that segregate as an autosomal dominant condition. The first family is a three-generation pedigree with 10 family members affected with congenital defects of the cardiac valves, including six patients with aortic stenosis and/or aortic regurgitation. Pulmonary and/or tricuspid valve abnormalities were present in three patients, and ventricular septal defect (VSD) was present in two patients. The second family consists of 11 patients in three generations with aortic valve stenosis in seven patients, defects of the pulmonary valves in two patients, and atrial septal defect (ASD) in two patients. Incomplete penetrance was observed in both families. Although left-ventricular outflow tract obstruction was present in most family members, the co-occurrence with pulmonary valve abnormalities and septal defects in both families is uncommon. These families provide evidence that left-sided obstructive defects and thoracic aortic aneurysm may be accompanied by right-sided defects, and even septal defects. These families might be instrumental in identifying genes involved in cardiac valve morphogenesis and malformation. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Anomalies of the atrioventricular and semilunar heart valves and associated structures account for 25–30% of all congenital cardiovascular malformations (CVM) [Loffredo, 2000]. Most occur sporadically in a single patient without affected family members, and unassociated with other malformations (nonsyndromic). On the other hand, well-defined syndromes with autosomal dominant inheritance, such as Noonan syndrome (caused by PTPN11, KRAS, SOS1, or RAF1 mutations), and Alagille syndrome (caused by JAGGED1 and NOTCH2 mutations) are often associated with valve defects [McElhinney et al., 2002; McDaniell et al., 2006; Sznajer et al., 2007].
Only a limited number of families with clear monogenic inheritance of nonsyndromic forms of congenital valve defects have been described. Familial nonsyndromic valve anomalies often include either the left-sided heart valves (aortic and mitral valve) or the right-sided heart valves (tricuspid and pulmonary valve). Left-sided valve anomalies can be part of a spectrum of anomalies of the left-ventricular outflow tract referred to as LVOTO (left-ventricular outflow tract obstruction; also known as obstructive anomalies of the left-heart and aorta) [McBride et al., 2005; Wessels et al., 2005; Garg et al., 2006], but in some families they occur without other LVOTO anomalies [Rao et al., 1969; McDonald and Maurer, 1989; Menahem, 1990]. In two families with LVOTO anomalies including bicuspid aortic valve (BAV) with calcification, Garg et al. 2006 documented truncating mutations in NOTCH 1. Two patients in these families also exhibited right-sided heart malformations, including double outlet right-ventricle and tetralogy of Fallot. Bicuspid aortic valve underlies the majority of patients with aortic valve disease and familial BAV has been described in single families suggesting autosomal inheritance [Emanuel et al., 1978; Glick and Roberts, 1994; Clementi et al., 1996; Huntington et al., 1997]. Studies on heritability of BAV support that genetic factors play a major role in BAV and demonstrate that BAV is often associated with other cardiovascular malformations, in particular LVOTO anomalies and thoracic aortic aneurysm (TAA) [Cripe et al., 2004; McBride et al., 2005; Loscalzo et al., 2007]. Locus heterogeneity for familial BAV has been established in several studies [Goh et al., 2002; Ellison et al., 2007; Martin et al., 2007], and genome-wide scans in families with BAV and/or associated CVM has demonstrated linkage to chromosomes 15q25-26, 18q, 5q, and 13q [Goh et al., 2002; Martin et al., 2007].
Another frequent left-sided heart valve anomaly, mitral valve prolapse, can be inherited as an autosomal dominant trait, and has been linked to chromosome 13 [Freed et al., 2003; Nesta et al., 2005]. Familial right-sided valve anomalies frequently represent syndromic forms of CVM and only a few nonsyndromic families are reported. Pulmonary stenosis (PS) is common in families with Noonan syndrome (PTPN11 mutations), Watson syndrome (NF1 mutations), and Alagille syndrome (JAGGED1 mutations). Mutations in PTPN11, however, have not been convincingly shown to be present in patients with nonsyndromic PS [Sarkozy et al., 2003], although a few cases of possible nonsyndromic PS with JAGGED1 mutations have been described [Krantz et al., 1999]. Nonsyndromic familial right-sided valve anomalies have only been described in a few small families. PS has been reported in some families with clear autosomal dominant inheritance [David, 1974; Ciuffo et al., 1985], and in some families with unknown mode of inheritance [Coblentz and Mathivat, 1952; Lamy et al., 1957; McCarron and Perloff, 1974; Klinge and Laursen, 1975; El-Said et al., 1979; Udwadia et al., 1996]. PS combined with ASD in a large pedigree has been shown to be due to a mutation in the GATA4 gene [Garg et al., 2003]. Familial pulmonic valve atresia and familial occurrence of tricuspid anomalies are very uncommon, and have only been described in a few families [DiChiara et al., 1980; Chitayat et al., 1992; Kumar et al., 1994; Grant, 1996; Grossfeld et al., 1997; Lin and Rosti, 1998; Bonnet et al., 1999].
Only a few families with combined left- and right-sided heart valve anomalies with monogenic inheritance have been described. A large family with autosomal dominant inheritance of mainly atrioventricular valve defects including Ebstein anomaly and atrioventricular canal has been described by Schunkert et al. 1997. Atrioventricular septal defects (AVSD) (also known as endocardial cushion defects) can be inherited as an autosomal dominant trait with variable expression and incomplete penetrance. These valve anomalies can be due to mutations in the gene encoding the cell adhesion molecule CRELD1 [Robinson et al., 2003], and a second locus is located on chromosome 1p31-p21. A few families with X-linked valvular dysplasia, a condition characterized by myxomatous degeneration, valvular regurgitation and secondary calcification affecting all four heart-valves have been described [Newbury-Ecob et al., 1993; Kyndt et al., 1998]. Recently mutations in the FLNA gene encoding filamin A were identified in these families [Kyndt et al., 2007].
The paucity of multiplex families with a clear Mendelian inheritance pattern of nonsyndromic cardiac valve malformation has precluded the identification of human genes specifically involved in cardiac valve morphogenesis and malformation. We describe two families with a similar autosomal dominant form of congenital heart malformation mainly consisting of cardiac valve anomalies.
CLINICAL REPORTS
Family 1
The pedigree of family 1 is shown in Figure 1.
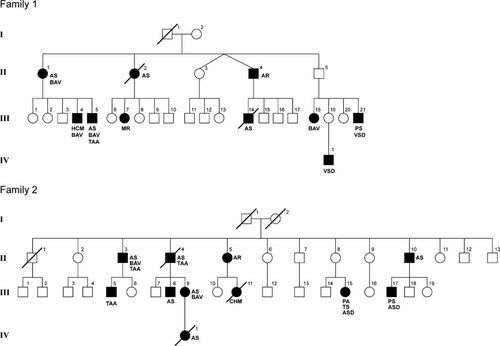
Pedigrees of two families with autosomal dominant inheritance of congenital valve anomalies. AR, aortic valve regurgitation; AS, aortic valve stenosis; ASD, atrial septal defect; BAV, bicuspid aortic valve; CVM Cardiovascular malformation; HCM, hypertrophic cardiomyopathy; MR, mitral valve regurgitation; PS, pulmonary valve stenosis; PA, pulmonary atresia; TAA, thoracic aortic aneurysm; VSD, ventricular septal defect.
Patient 1
The index patient (II-1) visited our Department of Clinical Genetics for genetic counseling. She had aortic valve replacement at the age of 42 because of a severely stenotic BAV. She received two new aortic valve prostheses in the following 30 years, and at the age of 60 she developed atrial fibrillation. Three of the five children of patient II-1 were healthy, and cardiologic examination including ECG and echocardiography revealed no abnormalities. Two other children (Patients 2 and 3) are affected.
Patient 2
One of the five children (III-4) of Patient II-1 was asymptomatic until he presented with progressive dyspnea at the age of 44. Echocardiography revealed a BAV, a dilated left-ventricle with thickening of the posterior wall, and left-ventricular dysfunction.
Patient 3
Another son (III-5) of Patient II-1 was diagnosed with a BAV with mild stenosis and regurgitation, and mild dilatation of the aorta at the age of 26 years. An X-ray of the thorax showed an elongated aorta. At the age of 36 years the aortic valve was stenotic, calcified and thickened, and mild tricuspid regurgitation was present. His left-ventricular function remained good. Chromosomal analysis showed a normal male karyotype. A microdeletion of the 22q11 (TBX1 gene) and 7q11.23 (elastin gene) region was excluded by fluorescent in situ hybridization (FISH).
Patient 4
A sister (II-2) of Patient II-1 was diagnosed with valvular aortic stenosis and regurgitation, mitral stenosis with regurgitation, and tricuspid regurgitation. She underwent three operation for aortic and mitral valve prostheses between the age of 40 and 44 years. At the age of 45 years a tricuspid valve correction was performed. She died at the age of 50. In four of her five children cardiologic examination including ECG and echocardiography revealed no abnormalities.
Patient 5
A daughter (III-7) of Patient II-2 was diagnosed with moderate mitral valve regurgitation at the age of 50 years.
Patient 6
A brother (II-4) of Patient II-1 was diagnosed with aortic valve regurgitation and received an aortic valve replacement.
Patient 7
The son (patient III-14) of Patient II-4 was diagnosed with aortic stenosis. Surgical correction was performed at the age of 9 years. He died 1-day after surgery.
Patient 8
One of the three daughters of Patient II-5 (patient III-18) underwent valvulotomy for a severely stenotic BAV at the age of 9 years. She also received a mitral valve prosthesis for severe mitral valve regurgitation.
Patient 9
The brother of Patient III-18 (patient III-21) was operated at the age of 5 years for a VSD and valvular PS. He developed a re-stenosis and pulmonary valve regurgitation.
Patient 10
A son (IV-1) of an asymptomatic sister of Patient III-18 was diagnosed with a perimembranous VSD at the age of 4 months.
Family 2
The pedigree of family 2 is shown in Figure 1.
Patient 1
The parents of Patient (III-15) visited our Department of Clinical Genetics for genetic counseling when their daughter was born with pulmonary atresia with intact ventricular septum, double-chambered right ventricle an ASD. The aortic valve showed no abnormalities. Surgical correction was performed in the first year and again at the age of 5 years. At the age of 7 years an obstructive fibromuscular bundle in the right ventricle was removed, and a small tricuspid valve with prolapse of the leaflets was found during operation. The chordae were abnormally long and attached directly to the ventricle wall without papillary muscles. Chromosomal analysis showed a normal female karyotype. A microdeletion of the 22q11 region and 7q11.23 region were excluded by FISH. Cardiac evaluation of both parents of patient 1 showed no abnormalities, the asymptomatic mother (II-8) was 53 years at examination.
Patient 2
The son (III-17) of a brother of the mother of Patient III-15 was diagnosed at birth with a severe valvular and infundibular PS with intact ventricular septum and a secundum ASD. Surgical correction was performed at the age of 1-year. After a second operation at the age of 6 he died from hypoxic encephalopathy.
Patient 3
The father (II-10) of Patient III-17 underwent cardiologic evaluation at the age of 35 years because of the familial CVM, and a valvular aortic stenosis was diagnosed. Chromosomal analysis and FISH of the 22q11 region showed no abnormalities.
Patient 4
A brother (II-4) of Patient II-13 was diagnosed at the age of 31 years with a severe valvular aortic stenosis and regurgitation and dilatation of the ascending aorta. At the age of 32 years his severely calcified aortic valve was replaced by a Bjork Shiley prosthesis. Chromosomal analysis and FISH of the 22q11 region showed no abnormalities. He died suddenly at the age of 50 years.
Patient 5
The daughter (III-9) of Patient II-4 was evaluated at the age of 7 years for a cardiac murmur, but was thought to have no abnormality. After giving birth to an affected child (IV-1) she was re-evaluated and was diagnosed as having a stenotic BAV.
Patient 6
Patient III-9 gave birth to a girl (Patient IV-1) with critical valvular aortic stenosis who died several days after birth.
Patient 7
A son (III-8) of Patient II-4 was diagnosed with a severe valvular aortic stenosis and received aortic valve replacement.
Patient 8
A brother (II-3) of Patients II-4 and II-10 was examined at the age of 47 years because of the familial CVM: echocardiography revealed a mildly stenotic, thickened BAV, with mild dilatation of the ascending aorta. At the age of 58 years the aortic root diameter was 53 mm and there was an ascending aorta aneurysm measuring 61 mm. There was left-ventricular hypertrophy. A Bentall procedure including replacement of the ascending aorta and proximal aortic arch was performed. A severely calcified aortic valve was replaced. Pathological examination showed a calcified aortic valve and wall. Intima fibrosis was present and mild medial degeneration. The elastin fibers showed no abnormalities. The daughter of this patient (III-11) showed no abnormalities on cardiologic examination, including echocardiography.
Patient 9
A son (III-5) of Patient II-3 was examined by the cardiologist and had mild dilatation of the ascending aorta (43 mm) without valvular abnormalities, left ventricular dysfunction or hypertrophy.
Patient 10
A daughter (III-11) of a sister (II-5) of Patients II-8 died 12 days after birth with an enlarged heart.
Patient 11
Cardiologic evaluation of the mother (II-5) of patient 10 at the age of 43 years revealed no abnormalities, but re-evaluation at the age of 57 years showed a sclerotic aortic valve with moderate regurgitation.
Additional family members
Cardiologic evaluation (echocardiography and ECG) of healthy family members in generation II (II-2, II-6, II-7, II-8, II-9 and II-11) showed no abnormalities. These healthy family members were between 39 and 64 years of age at the time of examination. The grandparents in generation I were not investigated. The grandfather died of a cardiac arrest at the age of 57 years.
MOLECULAR STUDIES
Linkage analysis using polymorphic microsatellites D9S1826, D9S158, and D9S1838 flanking the NOTCH1 gene was performed in both families. This excluded NOTCH1 as the disease gene in both families since multiple recombinants were found. Additionally, sequence analysis of all coding exons and intron–exon boundaries of the NOTCH1 gene was normal in one affected patient in each family.
DISCUSSION
We describe two families with autosomal dominant inheritance of isolated CVM mainly involving the cardiac valves. None of the affected family members showed signs of a connective tissue disorder or malformation syndrome. Among the 10 affected family members of the first family, 6 were diagnosed with an abnormal stenotic and/or insufficient aortic valve. In three patients, regurgitation of the mitral valve was present. Pulmonary valve abnormality was diagnosed in one family member. In the second family, abnormal semilunar valves were present in nine family members, seven with abnormal aortic valves, and two with defects of the pulmonary valves (Table I). Different cardiologists evaluated patients and BAV may not always be reported if present. In both families, septal defects were present in several patients, which we believe may be part of the spectrum since they co-existed with valve anomalies in several family members. In the first family, one patient had a VSD and PS. In the second family, an ASD was present in a patient with PS and in a patient with pulmonary atresia. No other congenital abnormalities or dysmorphic features were present in any of the patients, indicating that the CVM in these families is nonsyndromic. In both families, autosomal dominant inheritance is well supported since there are three affected generations with male–male inheritance and expression in both females and males. Nonpenetrance is present in one obligate carrier in both families. In family 2, patient II-2 was unaffected at the age of 43 years but showed moderate aortic valve regurgitation 14 years later at the age of 57. Patient II-8 showed no abnormalities at echocardiography at the age of 53. She has a tri-leaflet aortic valve and normal function of cardiac valves and the left-ventricle.
| Family 1 | Family 2 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Anomaly | II-1 | III-4 | III-5 | II-2 | III-7 | II-4 | III-14 | III-18 | III-21 | IV-1 | III-15 | III-17 | II-10 | II-4 | III-9 | IV-1 | III-8 | II-3 | III-5 | III-11 | II-5 |
| Aortic stenosis | + | + | + | + | + | + | + | + | + | + | + | ||||||||||
| Bicupid aortic valve | + | + | + | + | + | + | |||||||||||||||
| Thoracic aortic aneurysm | + | + | + | + | |||||||||||||||||
| Aortic regurgitation | + | + | + | + | |||||||||||||||||
| Pulmonary atresia | + | ||||||||||||||||||||
| Pulmonary stenosis | + | + | |||||||||||||||||||
| Mitral stenosis | + | ||||||||||||||||||||
| Mitral regurgitation | + | + | + | ||||||||||||||||||
| Tricuspid stenosis | + | ||||||||||||||||||||
| Tricuspid regurgitation | + | ||||||||||||||||||||
| Atrial septal defect | + | + | |||||||||||||||||||
| Ventricular septal defect | + | + | |||||||||||||||||||
| Cardiomyopathy | + | ||||||||||||||||||||
| Unspecified CVM | + | ||||||||||||||||||||
Autosomal dominant inheritance of nonsyndromic congenital valve anomalies has only been described in a limited number of families. In most cases consistence of valve anomalies of either predominantly left- or right-sided structures of the heart is present. In both presented families predominantly aortic valve anomalies were observed, although right-sided malformations such as pulmonary and tricuspid valve anomalies were present in some patients. This observation is also documented in other studies with smaller families (for instance only two persons affected) where patterns of inheritance are not always clear [Gill et al., 2003; Lewin et al., 2004; Martin et al., 2007]. In a recent study demonstrating high heritability of hypoplastic left-heart syndrome (HLHS) a high percentage of HLHS probands had both left- and right-sided valve dysplasia suggesting that HLHS is a severe form of valve malformation and anomalies of the left- and right-sided valves may have a common etiology [Hinton et al., 2007].
Human genes known to be involved in valvulogenesis include genes associated with elastogenesis and collagen synthesis, as elastin and collagen are major components of semilunar and atrioventricular valves. As mutations in most of these genes are associated with syndromic forms of CVM they are unlikely to be involved in our families with nonsyndromic CVM. Familial BAV and aortic valve stenosis in association with TAA is a well-recognized entity. Loscalzo et al. 2007 suggested that altered TGFB signaling might play a role in BAV with TAA as several aneurysm syndromes, including Marfan syndrome [Neptune et al., 2003], Loeys Dietz syndrome [Loeys et al., 2005], and arterial tortuosity syndrome [Coucke et al., 2006] are associated with upregulation of the TGFB pathway leading to loss of elastic fiber integrity. However, TGFBR1 and TGFBR2 gene analysis in 13 families with BAV and TAA revealed no mutations in [Loscalzo et al., 2007]. Recently mutations in the gene ACTA2, encoding vascular smooth muscle cell α- actin, were identified as a major cause of autosomal dominant inherited TAA. Interestingly multiple familymembers in 4 out of 14 described families with ACTA2 mutations showed BAV, indicating that genes encoding sacromeric proteins might be good candicate genes for a subset of familial LVOTO [Guo et al., 2007].
Elastin mutations in our families are unlikely as they predominantly cause supravalvular aortic stenosis, although aortic valve stenosis can also occur. Furthermore, right-sided valve anomalies and ASD/VSD are rarely described in patients with elastin mutations [Metcalfe et al., 2000; Eronen et al., 2002].
Only a few genes have been involved in nonsyndromic CVM with a monogenic mode of inheritance; these include the NOTCH1, Elastin, NKX2.5, NKX2.6, GATA4, CRELD1, MYH6, ACTA0, TBX20, and FLNA genes [for reviews: Bruneau, 2008; Ransom and Srivastava, 2007; Weismann and Gelb, 2007]. Mutations in NOTCH1 have been found in two families with BAV, aortic valve stenosis, aortic valve calcification and other LVOTO anomalies [Garg et al., 2006]. Interestingly one patient with AS and BAV also had ascending aortic dilatation. In both our families NOTCH1 was excluded as the disease gene by linkage analysis using polymorphic microsatellites (D9S1826, D9S158, D9S1838) flanking the NOTCH1 gene that showed multiple recombinants in both families. Additionally, sequence analysis of all coding exons and intron–exon boundaries was normal in an affected family member in both families.
Human NKX2.5 mutations can cause a number of different cardiac phenotypes [McElhinney et al., 2003; Elliott et al., 2003], including ASD/VSD and LVOTO, but anomalies of the semilunar valves, as observed in 17/21 of the patients in our families, are not often described [Majumdar et al., 2006]. A NKX2.5 mutation was excluded in both our families by mutation analysis in one affected family member. GATA4 mutations can lead to nonsyndromic ASD and other CVM including PS [Garg et al., 2003], whereas gross deletions of the 8p23 region encompassing the GATA4 gene are associated with a variety of cardiac anomalies, mainly PS and ASD. However, in contrast to our families left-sided cardiac anomalies are uncommon in these patients, although aortic/mitral regurgitation was reported in 1/18 patient of the families described by Garg et al. 2003. So far, no good candidate genes have been reported for our families, therefore a genome-wide linkage analyses has been initiated in both families.
Mature valve structures arise from endothelial cells of the endocardial cushions [Lincoln et al., 2004]. The endocardial cushions are formed by endothelial–mesenchymal transdifferentiation of a subset of endothelial cells that invade the extracellular matrix and differentiate into mesenchymal cells [Armstrong and Bischoff, 2004]. Valve leaflets eventually consist of a single endothelial cell layer and a central layer consistent of collagen, elastin, and glycosaminoglycans [Maron and Hutchins, 1974]. The endocardial cushion tissue contributes not only to the formation of valves, but also to the formation of membranous septa [Schroeder et al., 2003]. This might explain why some patients in our families have septal defects apart from valve anomalies.
In contrast to the sparse knowledge about the genes involved in human valve formation and malformation much more is known about valvulogenesis in mice. In mice signal transduction pathways including Wnt/β–catenin, Vegf, Notch, Bmp–Tgfβ, and Erb, and transcription factors including different GATA, FOX and SOX transcription factors have been implicated in heart cushion/valve formation (Table II, Fig. 2). These different signaling pathways exhibit extensive cross-talking, resulting in a complex integrated process of cardiac valve morphogenesis [for review: Schroeder et al., 2003; Armstrong and Bischoff, 2004]. These mouse models could provide functional candidate genes for families with cardiac valve anomalies once positional genetics approaches have localized the human disease gene.
| Gene | Cardiac anomaly | References |
|---|---|---|
| Wnt/β-Catenin signaling | ||
| Has2 | Absence of cardiac jelly/endocardial cushions |
Camenisch et al. 2000 |
| Hdf (Cspg2, versican) | Absence of endocardial cushion swelling |
Mjaatvedt et al. 1998 |
| β-catenin | Lack of heart cushion formation |
Liebner et al. 2004 |
| Notch signaling | ||
| Notch1 | Hypoplastic cardiac cushions |
Timmerman et al. 2004 |
| Hesr2 | Dysplastic AV valves, ASD, VSD |
Kokubo et al. 2004 |
| Hey 1/HeyL | Dysplastic atrioventricular and pulmonary valves |
Fischer et al. 2007 |
| Hey2 | TA, VSD, TOF |
Donovan et al. 2002 |
| EphrinB2 | Thickened aortic, pulmonary and mitral valve |
Cowan et al. 2004 |
| Fgf8 | Single AV valve, hypoplastic arch arteries, DORV |
Abu-Issa et al. 2002 |
| Ece1/Ece2 | Abnormal AV valve formation, truncus arteriosus |
Yanagisawa et al. 2000 |
| Vegf signaling | ||
| Cx45 | Endocardial cushion defects |
Kumai et al. 2000 |
| Nfatc1 | Absent semilunar valves |
Ranger et al. 1998 |
| Nf1 | Hyperplastic valve tissue |
Lakkis and Epstein 1998 |
| Tie 2 (TEK) | Endocardial cushion defects |
Puri et al. 1999 |
| eNos | BAV, AS, ASD, VSD |
Lee et al. 2000 |
| Hhex | AV valve dysplasia |
Hallaq et al. 2004 |
| Bmp-Tgf-β signaling | ||
| Bmpr2 | Absent semilunar valves, truncus arteriosus |
Delot et al. 2003 |
| Bmp4 | Variable |
Winnier et al. 1995 |
| Bmp6/7 | Hypoplastic cardiac cushions |
Kim et al. 2001 |
| Alk3 | Hypoplastic cardiac cushions |
Gaussin et al. 2002 |
| Madh6 (Smad6) | Thickened valves |
Galvin et al. 2000 |
| Perlecan (HSPG2) | Malformed semilunar valves, TGA |
Costello et al. 2002 |
| Fibulin-4 | Thickened aortic valvular leaflets |
Hanada et al. 2007 |
| Erb signaling | ||
| ErbB1 (Egfr, Her1) | Enlarged thickened semilunar and AV valves | |
| ErbB3 (Her3) | Hypoplastic cardiac cushion |
Erickson et al. 1997 |
| HB-EGF | Enlarged malformed semilunar and AV valves | |
| Tace (Adam17) | Enlarged semilunar and AV valves |
Jackson et al. 2003 |
| β Meltrin (Adam19) | Immature valves, VSD | |
| Egfr/Ptpn11 | Semilunar valve hyperplasia |
Chen et al. 2000 |
| GATA transcription factors | ||
| GATA4 | Common AV valve |
Crispino et al. 2001 |
| Fog 1 | Common AV valve, DORV |
Katz et al. 2003 |
| Fog 2 | TA, PS, AV canal, ASD, VSD, TOF | |
| Nkx2.5 | BAV, AS, ASD |
Biben et al. 2000 |
| Pitx2 | Enlarged endocardial cushion |
Lin et al. 1999 |
| Sox transcription factors | ||
| Sox4 | Semilunar valve defects, truncus arteriosus |
Ya et al. 1998 |
| Sox9 | Hypoplastic endocardial cushions |
Akiyama et al. 2004 |
| Fox transcription factors | ||
| Foxp1 | Thickened endocardial cushion |
Wang et al. 2004 |
| Foxc1 | Valve anomalies, IAA, CoA, VSD |
Winnier et al. 1999 |
| Foxc2 | Valve anomalies, IAA, CoA, VSD |
Winnier et al. 1999 |
| Various | ||
| EphA3 | Hypoplastic endocardial cushions |
Stephen et al. 2007 |
| Pdgf-a(patch mutation) | Septal and valve defects |
Robbins et al. 1999 |
| Pdgf-b | AV valve malformation, VSD |
Van den Akker et al. 2008 |
| Apoe | Sclerotic, stenotic aortic valves |
Tanaka et al. 2005 |
| Ccn1 | Atrioventricular septal defects |
Mo and Lau 2006 |
| Periostin | AV valve abnormalities, ASD |
Norris et al. 2008 |
| Chm1 | Thickened, calcified, stenotic aortic valves |
Yoshioka et al. 2006 |
| Cxcr7 | Semilunar valve malformation, VSD |
Sierro et al. 2007 |
- AV, atrioventicular; AS, aortic valve stenosis; ASD, atrial septal defect; BAV, bicupid aortic valve; CoA, coarctation of the aorta; DORV, double outlet right ventricle; IAA, interrupted aortic arch; PS, pulmonary stenosis; TA, tricupid atresia; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
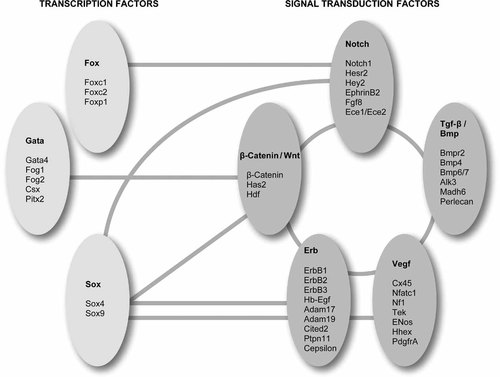
Molecular pathways and their cross-talking involved in cardiac morphogenesis. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The two multiplex families in this report may facilitate identification of human genes specifically involved in cardiac valve morphogenesis and aortic wall disease. The co-occurrence of aortic and pulmonary valve abnormalities and aortic aneurysms in these families supports a common genetic etiology in some forms of left- and right-sided valve anomalies, and expands the phenotype of LVOTO.




