Somatic TP53 mutation mosaicism in a patient with Li–Fraumeni syndrome†
How to cite this article: Prochazkova K, Pavlikova K, Minarik M, Sumerauer D, Kodet R, Sedlacek Z. 2009. Somatic TP53 mutation mosaicism in a patient with Li–Fraumeni syndrome. Am J Med Genet Part A 149A:206–211.
Abstract
We present a girl who developed adrenocortical adenoma at the age of 1 year and osteosarcoma at the age of 5 years. There was no history of cancer in her parents and their relatives. However, both tumors were typical for the Li–Fraumeni syndrome (LFS), and the patient met criteria for germline TP53 mutation testing. A mutation in codon 282 (Arg282Trp) was identified in her blood lymphocyte genomic DNA. The substitution was found in neither of her parents, which indicated a possibility of a de novo mutation. Unexpectedly, sequencing of the DNA of the patient repeatedly showed allelic imbalance in favor of the normal allele. This observation prompted us to investigate the putative somatic mosaicism in the patient consisting of normal cells and cells heterozygous for the mutation. The imbalance was also examined in two other non-invasively sampled tissues, buccal cells, and cells from the urine sediment, and sequencing was confirmed with two other independent methods. While the findings in blood and the urine sediment were similar, in buccal cells both alleles were present in equal amounts. The allele ratio in lymphocytes was consistent with a mosaic where about 2/3 of cells carried two normal alleles and only 1/3 was heterozygous for the mutation. Despite the mosaicism the girl developed two early childhood tumors of mesodermal origin, and her phenotype was thus not milder than that of other germline TP53 mutation carriers. To our knowledge this is the first description of somatic mosaicism for a de novo TP53 mutation in LFS. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Li–Fraumeni syndrome (LFS) is a hereditary cancer predisposition syndrome characterized by an increased predisposition to a broad range of tumors. Sarcomas, brain tumors, and breast cancer are diagnosed most frequently in these patients, but many other types of tumors may be observed. Many patients are affected in early childhood, most often by sarcomas, brain and adrenocortical tumors. Multiple primary tumors also occur very often in these individuals [Li and Fraumeni, 1969]. The lifetime risk of developing cancer reaches about 73% for men and nearly 100% for women [Frebourg et al., 2001]. The diagnosis of LFS is based on strict clinical criteria [Li et al., 1988]. Germline mutations in the TP53 gene are found in about 70% of families conforming to these criteria [Malkin et al., 1990]. However, there are many families with remarkable hereditary predisposition to cancer suggestive of LFS who do not meet the stringent diagnostic criteria, and more relaxed criteria have been proposed for TP53 mutation testing, for example, the Li–Fraumeni-like [Birch et al., 1994; Eeles, 1995] or the Chompret criteria [Chompret et al., 2001]. In families meeting these less strict criteria, TP53 gene mutations can be identified in <20% of cases. The carriership of a germline TP53 mutation represents a severe burden for the affected individuals, and this genetic defect has a devastating effect on their families. Similar to other autosomal dominant genetic defects with impaired fitness, selection is likely to operate against the constitutional TP53 mutations, and the frequency of the disorder in the population is likely to be maintained by influx of new mutations. Indeed, de novo germline TP53 mutations have been reported in several individuals [Sedlacek et al., 1998b]. In this report, we present a girl with two tumors typical for LFS but no family history of cancer. A mutation was identified in the child but in none of her parents. Further analysis indicated that the girl was a somatic mosaic for a de novo TP53 mutation, with different ratios of normal and mutated alleles in different tissues. To our knowledge this is the first description of a clear post-zygotic origin of a de novo constitutional TP53 mutation, leading to somatic mosaicism and cancer predisposition.
CLINICAL REPORT
The patient was a 7-year-old girl with a significant history of cancer. At the age of 1 year she presented with a virilizing adrenocortical tumor, which was completely resected. The histopathological analysis confirmed adrenocortical adenoma. The girl was then monitored by the endocrinology service and had no complaints until 5 years of age when she presented with a firm mass below the left knee, after a 1-week history of limping and pain without any previous trauma. Diagnostic imaging and biopsy confirmed the diagnosis of osteosarcoma of the left proximal tibia. Chest radiography, abdominal ultrasound, computerized tomography of the chest, abdomen and pelvis, and bone scan were normal. The girl was treated with adjuvant chemotherapy consisting of cisplatin, doxorubicine, and high-dose methotrexate, and a limb-sparing surgery. The histopathological evaluation of the resected specimen confirmed wide tumor resection and an excellent response to preoperative chemotherapy. The girl was treated with postoperative chemotherapy, which was completed within 32 weeks of the diagnosis. At present, 30 months later, she is in complete remission. The history of cancer in the family was negative with a possible exception of leukemia at 33 years of age in one of six siblings of the maternal grandmother (Fig. 1). However, the occurrence of two tumors typical for LFS was strongly suggestive of a genetic predisposition. The patient did not meet the criteria for LFS [Li et al., 1988] but met the Chompret criteria for TP53 mutation testing [Chompret et al., 2001]. The family was offered genetic counseling and decided for TP53 testing.
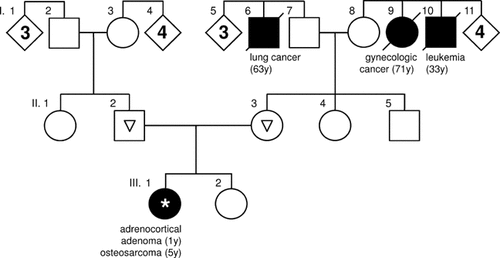
Pedigree of the patient's family showing no remarkable history of cancer. Family members affected by tumors are represented by black filled symbols; diamonds with numbers indicate multiple unaffected siblings of any sex. Age at onset of the tumor in years is given in parentheses. Asterisk, confirmed carriership of the TP53 mutation in the patient; triangles, absence of the mutation in her parents.
MATERIALS AND METHODS
DNA Samples
Lymphocyte genomic DNA was isolated from two independent blood samples from the patient and her parents. Genomic DNA from blood and from the urine sediment of the patient was extracted using the Gentra Puregene Blood Kit and the QIAamp DNA Mini Kit (both from Qiagen, Hilden, Germany), respectively. DNA from buccal cells was extracted using the JetQuick Tissue DNA Isolation Kit (Genomed, Loehne, Germany). DNA from a patient with a germline TP53 mutation in codon 281 [Krutilkova et al., 2005], two DNA samples from esophageal tumors with various fractions of somatic TP53 mutations in codon 282 [Novotna et al., 2006], and DNA from an individual with no TP53 mutation were used as controls.
PCR Amplification and Sequencing
Exons 1–11 of the TP53 gene were amplified from the DNA of the patient. The amplification of exons 5–9 was described previously [Sedlacek et al., 1998a], other primers and conditions used in this work are available from the authors upon request. The PCR products were purified using the QIAamp PCR Purification Kit (Qiagen) and sequenced from both DNA strands using the PCR primers on an ABI PRISM 3100 automatic sequencer (Applied Biosystems, Foster City, CA). The sequences were analyzed using Staden Package software (http://sourceforge.net/projects/staden).
Denaturing Gradient Gel Electrophoresis (DGGE)
A separate PCR of exon 8 was performed using primers with a CG clamp under conditions described previously [Hamelin et al., 1993]. Heteroduplexes were formed by denaturation and slow cooling of the samples. Denaturing gradient gel electrophoresis (DGGE) was performed as described [Hamelin et al., 1993] using a denaturing gradient between 20% and 70%. The gel was stained and documented using GelCapture software (DNR Bio-Imaging Systems, Jerusalem, Israel).
Preparation of Artificial Equimolar Mixture of Normal and Mutated Alleles
The bands representing both types of heteroduplexes (each containing one normal and one mutated DNA strand) were excised from the DGGE gel. DNA was eluted by diffusion into EB buffer (Qiagen) for 24 hr, and re-amplified using a fluorescein-labeled and a GC clamp-containing primer.
Cycling Gradient Capillary Electrophoresis (CGCE) and Quantification of Allele Ratios
The artificially prepared equimolar mixture of normal and mutated alleles as well as amplified blood and buccal cell DNAs of the patient were analyzed using cycling gradient capillary electrophoresis (CGCE) as described previously [Minarik et al., 2003]. The areas under the peaks of the homoduplexes of normal and mutated alleles were obtained from five different runs of each sample, and the allele ratios were normalized to the peak ratios of the equimolar mixture. This subsequently allowed the calculation of the ratio of homozygous and heterozygous cell lines in the samples.
RESULTS
A missense mutation CGG to TGG (Arg282Trp) in exon 8 of the TP53 gene was identified in blood genomic DNA of the patient. Repeated sequencing of both DNA strands from two independent blood samples yielded a reproducibly weaker signal from the mutated allele compared to that from the normal allele (Fig. 2). None of the parents of the patient tested positive for the mutation. These findings led us to the notion that the patient might be a somatic mosaic for a de novo TP53 mutation. Classical cytogenetic examination showed normal karyotype (data not shown) and ruled out mosaicism due to a chromosome aberration.
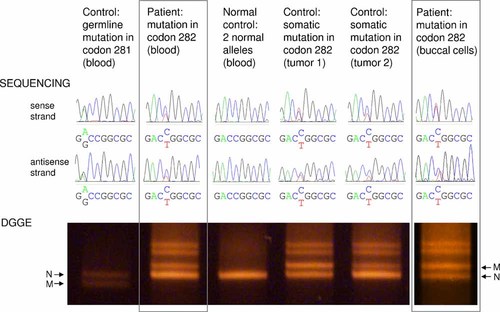
Sequencing of codons 281–283 and DGGE analysis of exon 8 of the TP53 gene in two tissues of the patient (framed) and four controls. Controls: a patient with GAC to GGC (Asp281Gly) germline TP53 mutation, an individual with no TP53 mutation, and two tumors with various ratios of somatic CGG to TGG (Arg282Trp) TP53 mutations (identical to the mutation in the patient). Sequencing results from both DNA strands are presented and allelic imbalance is indicated by unequal peak heights/areas. The DGGE gel shows homoduplexes (lower bands, showing allelic imbalance) and heteroduplexes (upper bands, where the allele ratio is always 1:1). N, normal allele; M, mutated allele.
The DGGE analysis confirmed the allelic imbalance observed in DNA sequencing: the band of the normal homoduplex was repeatedly significantly stronger than the band of the mutated homoduplex (Fig. 2). In contrast, the control sample from a patient with a germline TP53 mutation in neighboring codon 281 showed equal allele ratios using both methods, indicating only heterozygous cells as the source of the DNA (Fig. 2). Good correlation was also observed between sequencing and DGGE in two DNAs from esophageal tumors carrying various proportions of somatic Arg282Trp TP53 mutations. While tumor 1 showed an equal ratio of both alleles using both methods, the ratio of the mutated and normal allele was lower in DNA from tumor 2 (Fig. 2). This was similar to blood DNA of the patient and suggested a mixture of normal cells and cells heterozygous for the mutation as the source of the DNA.
As a next step we examined other tissues of the patient. Sequencing of DNA from the urine sediment indicated allelic imbalance similar to that in blood (data not shown). In contrast, in buccal cells both alleles were present in approximately equal amounts, both in sequencing and DGGE (Fig. 2). These differences observed among different tissues further supported the notion that the patient could be a somatic mosaic for a de novo TP53 mutation.
To confirm the above findings by a third independent method and to quantify the allele and cell line ratios, we analyzed the artificial equimolar mixture of both alleles in parallel with lymphocyte and buccal cell DNAs from the patient by CGCE (Fig. 3). The quantification showed that while the allele ratio was about 1:1 (normal:mutated) in buccal cells, this value reached only 1:0.2 in blood lymphocytes. This was consistent with a mosaic where about 2/3 of blood lymphocytes carried two normal alleles and only 1/3 was heterozygous for the mutation, in contrast to the buccal tissue where all cells were heterozygous.
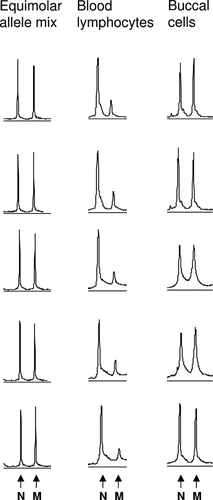
CGCE profiles of homoduplexes of normal (N) and mutated (M) alleles of TP53 exon 8 from an artificially prepared equimolar allele mix and from two tissues of the patient showing clear allelic imbalance in blood lymphocytes. Five independent runs of each sample are depicted. The average ratios of peak areas (N:M) were 1:0.79, 1:0.16, and 1:0.81 for the equimolar allele mix, blood lymphocytes, and buccal cells, respectively.
DISCUSSION
In genetic disorders in which sporadic cases are frequent, these sporadic patients often carry de novo mutations. A de novo mutation can arise either in a germ cell of one of the parents of the affected individual (pre-zygotic origin), or after fertilization in one of the cells of the embryo (post-zygotic origin) [Bernards and Gusella, 1994; Kluwe and Mautner, 1998]. De novo mutations can be associated with somatic mosaicism, the co-occurrence of a normal cell line, and a cell line carrying the mutation in one individual. Disregarding reversion of an inherited mutation, mosaicism can be associated only with post-zygotic de novo mutations.
De novo mutations in the TP53 gene are reported rarely in LFS individuals [Sedlacek et al., 1998b], in contrast to other disorders like neurofibromatosis type 1 where about 50% of cases are sporadic and are caused by de novo mutations in the NF1 gene [Lazaro et al., 1994]. However, de novo mutations may be underreported in LFS: there are currently 18 other patients with no family history of cancer and a TP53 mutation described in the literature, and in many of them the information on the parental mutation status is missing [Sedlacek et al., 1998b]. Reports on somatic mosaicism for constitutional TP53 mutations are even rarer. Only one instance of suspected germ line mosaicism was described in an unaffected female with no TP53 mutation in blood lymphocytes and two sons suffering from LFS and carrying identical germline TP53 mutations [Kovar et al., 1992]. To our knowledge, our patient thus represents the first case of a defined somatic mosaicism for a TP53 mutation analyzed at the DNA level.
The girl carried a missense mutation CGG to TGG in codon 282 of the TP53 gene. This mutation affecting a CG dinucleotide has already been described in eight unrelated LFS families [Sedlacek et al., 1998b]. The notion that our patient was a mosaic of normal cells and cells carrying the mutated TP53 allele was supported by several observations: the allelic imbalance present in blood as confirmed by three independent methods, the different ratios of the allelic imbalance in different tissues of the patient, and the absence of the mutation (and of a family history of cancer) in the parents of the girl. The latter could also result from non-paternity; however, in our case the scenario involved a post-zygotic origin of the mutation, and the actual paternity in the family was thus of no relevance.
It is known that direct DNA sequencing poorly reflects the representation of individual alleles in heterozygous or mixed samples [Fan et al., 2001]. Similarly, the images of the DGGE gels underwent several levels of processing, the optical density varied in different sectors of electrophoretic lanes, and the brighter bands were likely to be affected by saturation of the signal. For this reason, the final experimental confirmation of the mosaicism relied on CGCE, where the quantity of the alleles is proportional to the peak areas, and the area ratios thus yielded the most realistic estimate of the allele content.
In contrast to TP53, multiple patients with somatic mosaicism were reported for the NF1 [Lazaro et al., 1994; Colman et al., 1996; Consoli et al., 2005] and NF2 gene mutations [Kluwe and Mautner, 1998; Evans et al., 2005]. Mosaicism for a NF1 gene mutation can cause segmental neurofibromatosis 1 with a regionally limited distribution of the features of the disorder [Consoli et al., 2005]. Mosaicism for NF2 mutations was similarly proposed to cause a milder phenotype of neurofibromatosis 2 [Kluwe and Mautner, 1998]. Mosaicism was often described in other skin disorders where a clinically observable patchy distribution of the disease presentation correlated with mosaicism in the genotype of the cells [Paller et al., 1994; Hafner et al., 2006]. Similarly, mosaic constitutional mutations of the APC gene often lead to attenuated or atypical familial adenomatous polyposis [Aretz et al., 2007; Hes et al., 2008]. According to this view, our patient could, due to her mosaicism, present with a milder phenotype. However, the girl had suffered from two independent primary cancers before the age of 5 years, and her phenotype was therefore rather severe.
The tissues analyzed in our study arise during development from two different germ layers: ectoderm (buccal cells) and mesoderm (lymphocytes). The urine sediment can contain a mixture of cells of mesodermal and ectodermal origin. It is interesting that the mosaicism was very pronounced in blood lymphocytes and in cells from the urine sediment but could not be detected in buccal cells where both alleles were present in equal amounts, which indicated a strong prevalence or exclusive occurrence of the heterozygous cell line. Nevertheless, both tumors of the patient (adrenocortical adenoma and osteosarcoma) developed in tissues of mesodermal origin where the mutation was present at a reduced frequency.
Acknowledgements
We thank Dr. Drahuse Novotna for karyotyping of the patient. The work was supported by grants MSM0021620813 (Ministry of Education of the Czech Republic) and MZO00064203 (Ministry of Health of the Czech Republic).




