Poikiloderma with neutropenia, Clericuzio type, in a family from Morocco†‡
How to cite this article: Mostefai R, Morice-Picard F, Boralevi F, Sautarel M, Lacombe D, Stasia MJ, McGrath J, Taïeb A. 2008. Poikiloderma with neutropenia, clericuzio type, in a family from Morocco. Am J Med Genet Part A.
Rahima Mostefai and Fanny Morice-Picard contributed equally to this work.
Abstract
Three siblings from Morocco consanguineous family presented with cutaneous poikiloderma following postnatal ichthyosiform lesions, associated with papillomatous lesions, palmoplantar keratoderma, pachyonychia of toenails, fragile carious teeth, and lachrymal duct obstruction. Photosensitivity and blistering improved with age. Atrophic scars were prominent on the limbs. Neutropenia developed in the first year secondary to dysmyelopoiesis affecting the granulocyte lineage, associated with a polyclonal hypergammaglobulinemia. Several broncho-pulmonary infectious episodes complicated the evolution, and cystic fibrosis was first considered on the basis of repeated abnormal sweat chloride tests but not confirmed by molecular analyses. This autosomal recessive disorder matches that described originally as poikiloderma with neutropenia-Clericuzio type in Navajo Indians (OMIM 604173). It is discussed within the group of the major hereditary poikiloderma disorders, that is, Rothmund–Thomson syndrome, dyskeratosis congenita, and Kindler syndrome. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Hereditary poikilodermas comprise a group of rare disorders, the best known of which is Rothmund–Thomson syndrome (RTS) (OMIM # 268400), an autosomal recessive disorder with a heterogeneous clinical profile that includes a characteristic skin rash (poikiloderma), small stature, sparse hairs, skeletal, dental, and nail abnormalities, cataracts, and an increased risk of cancer (squamous cell carcinoma, osteosarcoma) [Starr et al., 1985; Vennos et al., 1992; Pujol et al., 2000; Wang et al., 2001]. Mutations of the human helicase gene RECQL4 located on human chromosome 8q.24.3 have been identified in a subset of patients with RTS (OMIM # 603780), [Kitao et al., 1999]. The RECQL4 protein belongs to the RECQ family of DNA helicases, also mutated in Bloom and Werner syndrome. We report on an autosomal recessive poikiloderma of early onset with a striking early ichthyosiform component, associated with chronic neutropenia and recalcitrant pulmonary disease initially diagnosed as cystic fibrosis on the basis of repeated abnormal sweat chloride tests. The disorder described in this family confirms the existence of a new genetic subtype of hereditary poikiloderma already named poikiloderma with neutropenia, Clericuzio type (OMIM 604173).
CLINICAL REPORTS
The family is composed of three affected siblings (two females, one male) and one unaffected sister born of healthy first cousin parents originating from Morocco (See family tree Fig. 1). The proposita was the eldest child born by vaginal delivery at term. Her medical history started when she was admitted at 4 months of age for a Haemophilus influenzae pulmonary infection. There were no other findings except a moderate splenomegaly. Respiratory manifestations remitted after symptomatic and antibiotic treatment. Ten months later, erythematous papules and plaques were noted on sun exposed areas, that is, face and dorsum of limbs associated with small brown ichthyotic squames (Fig. 2). At 30 months her skin had a mottled appearance with hypo and hyperpigmented macules, telangiectasias and atrophic spots arising on an erythematous background on photoexposed areas. Photosensitivity was confirmed by occasional blistering from the age of 2 years during the summer time in Morocco with formation of residual atrophic scars particularly on the dorsum of fingers, elbows and knees extending later to all extensor surfaces of the limbs. Blistering and photosensitivity tended to improve with age while the skin condition extended to the rest of the body without sparing palms and soles which appeared thickened (Fig. 3). Pachyonychia of the big toenails developed by the age of 3 years (Fig. 4). At that time, there was a slight growth delay (−1 SD for height and weight). A mild developmental delay was also noted. She did not walk until 21 months. By the age of 6 years, milia and keratotic papules developed on her face and forearms (Fig. 5a,b). Hair and mucous membranes were unaffected but fragile carious teeth were observed since the first teeth eruption with episodes of dental abscesses necessitating extraction (at age 7). Ophthalmologic examination revealed obstruction of the lachrymal ducts with continuous tears. Recurrent respiratory infections complicated her infancy and required repeated hospital admissions. The main cause identified was Hemophilus influenzae isolated repeatedly in nasopharyngeal aspirations. Pulmonary sequela developed by the age of 6 years and a severe restrictive respiratory syndrome with bronchiectasis and chronic interstitial lung infiltration was diagnosed. Other infectious complications included recurrent otitis media, facial cellulitis secondary to a dental abscess, and left inguinal adenitis with associated folliculitis of the buttocks. Table I shows the clinical findings in the other affected siblings who all had a strikingly similar clinical pattern.
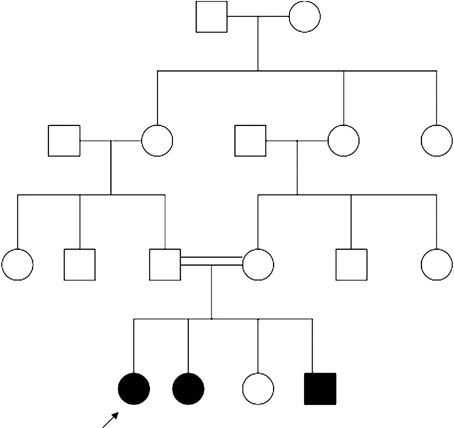
Family tree.
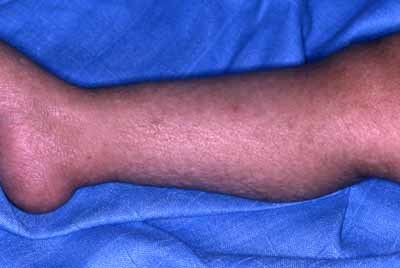
Brown ichthyotic squames of legs of brother at 3. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
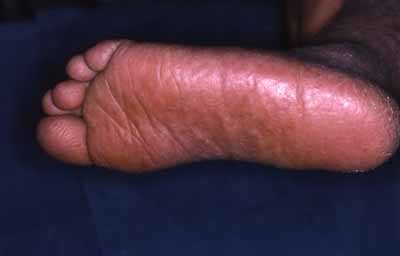
Thickened aspects of soles in proposita at 3. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
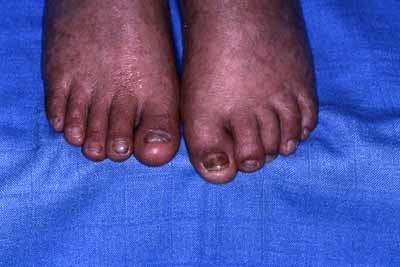
Pachyonychia of toenails in proposita at 3. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
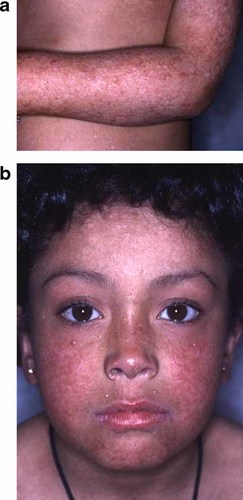
a: Aspects of poikiloderma on the volar aspect of arms in proposita at 7. b: Milia and keratotic papules of the face in sister at 8. Note the absence of dysmorphic features. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Proposita | Sister | Brother | |
|---|---|---|---|
| Skin manifestations | |||
| Onset | 14 M | 15 D | 6 M |
| Photosensitivity | ++ | ++ | + |
| Blistering | ++ | ++ | −/+ |
| Atrophic scars | +++ (dorsum of limbs) | +++ (dorsum of limbs) | + |
| Pachyonychia | Big toenails | Big toenails | − |
| PPK | + | + | − |
| Verrucous lesions | + | − | + |
| Milia | + | − | + |
| Carious teeth | + | + | + |
| Lachrymal duct obstruction | + | + | + |
| Pulmonary manifestations | Since 4 M RBP | Since 12 M RBP | Since 1 M RBP |
| Other infections | ROM, facial cellulitis, adenitis | ROM, folliculitis. | ROM, blepharitis |
| Mental and growth retardation | Mild MR | Moderate GR (−2 SD for H and W at 3 y of age) mild MR | Not assessed |
- D, days; GR, growth retardation; H, height; M, months; MR, mental retardation; PPK, palmoplantar keratoderma; RBP, recurrent broncho pneumonitis; ROM, recurrent otitis media; W, weight; Y, years.
Investigations
Leukopenia (2,600–5,930/mm3; normal values (NV) [4,000–10,000/mm3]) with neutropenia (220–884/mm3; NV [2,500–7,500/mm3]) developed from the age of 14 months, and never improved; a moderate anemia (9.1–12.0 g/L) with anisocytosis was associated. Platelet counts were normal or low normal. Immunologic investigations found normal total lymphocyte counts, but a slight decrease in CD4 T cells with a mildly decreased CD4/CD8 ratio. A polyclonal hypergammaglobulinemia was associated (IgG 15 g/L; N < 11.8 g/L, IgA > 2 g/L; NV < 1.65 g/L); C3 was also increased. Coagulation tests were normal. The electrophoresis of hemoglobin was normal and fetal hemoglobin was not increased (0.8%). Bone marrow smears showed an increased cellularity and morphologic abnormalities affecting the three myeloid lineages, including maturational defects of the granulocyte lineage with raised myeloblasts, a blockade at the myelocyte stage and an asynchronous maturation of the erythroblast lineage. Bone radiographs were normal. A moderate splenomegaly was found on abdominal ultrasonography. Repeated sweat chloride tests were in the range of 40 mmol/L (NV < 40 mmol/L). Chest radiographs showed a chronic infiltration in the middle lobe and lingula and bronchial dilatation. A progressive restrictive pattern was found on pulmonary function tests. Antibodies against basal lamina and intercellular antigens were absent, nuclear antibodies were positive at 1/250. A 4 mm punch skin biopsy specimen was taken from the arm and histology showed no specific features. Because of the blistering history, epitope mapping of the epidermal basal lamina was performed using cytokeratins 10 and 14, integrins α3, α6, β4, plectin, BP 230 and BP 180, uncein, laminins 5, 1, and α3, β3, and γ2 subunits, collagen IV and VIII antibodies. It was normal, but with an unusual presence of cytokeratin 14 within epidermal layers notably within the stratum corneum and granulosum. Furthermore, in the superficial dermis colloid bodies stained positively with anti IgM, cytokeratin 14 and collagen IV. HPV studies of the keratotic papules of the face using in situ hybridation and PCR sequencing, found HPV 57, which is usually associated with common warts (See Table II for a Summary).
| Proposita | Sister | Brother | |
|---|---|---|---|
| Hematology | |||
| Detection of anomalies | 14 M | 12 M | 6 M |
| Blood cell counts | Leukopenia (2,600–5,930/mm3) | Leukopenia (2,900–4,700/mm3) | Transient thrombocytopenia, 1 Mo (55,000–120,000/mm3) |
| neutropenia (220–884/mm3) | neutropenia (345–1,540/mm3) microcytic anemia | neutropenia (183–5,200/mm3) | |
| Electrophoresis Hb | Normal | Normal | Normal |
| Bone marrow smears | Myeloid lineage abnormal for morphology and maturation defects | Morphology abnormal and maturation defects in 3 cell lineages | Morphology abnormal. and maturation defects in 3 cell lineages |
| Immunology | |||
| Immunoglobulin profiles | Polyclonal [IgG, IgA=1,5-2No] | Polyclonal [IgG, IgA=2No] | Polyclonal [IgG, IgA=2,5No] |
| B Lymphocytes | No | No | No |
| T Lymphocytes | CD4/CD8 low No. | No | No |
| Imaging | |||
| Bone X-rays | No | No | No |
| Chest X-rays | CPI | CPI | CPI |
| Abdominal US | Splenomegaly | Splenomegaly | No |
| Sweat chloride test | 40 mmol/L | 60–80 mmol/L | 80–150 mmol/L |
| Molecular analyses | |||
| Karyotype | No | No | No |
| DNA UDS | No | No | Not analyzed |
| CFTR | Discordant | Discordant | Not analyzed |
| other | No mutation found | No mutation found | Not analyzed |
| (DKC1, TERC, RECQL4, KIND1) | |||
- BCC, blood cell counts; CPI, chronic pulmonary infiltrates; No, normal.
Cytogenetic and molecular analyses
Bone marrow was obtained for karyotype and sister-chromatid exchange studies, which were normal. Molecular analyses to excluded cystic fibrosis (CFTR mutations) were performed because of abnormal sweat chloride tests (Table II) and repeated pulmonary infections. The eight most frequent mutations of the CFTR gene (F508del, G542X, N1303K, 1717-1G > A, W1282X, G551D, R553X, I507del) were investigated in leukocyte DNA samples by reverse hybridization on specific PCR products, and were not identified. In addition, because of the strong clinical suspicion of CF diagnosis, a linkage analysis of three intragenic microsatellites of the CFTR gene (IVS8CA, IVS17b TA, IVS17bCA) was done, revealing that the proposita and her healthy sister shared the same CFTR haplotype. These data indicate that the CFTR gene is not linked to the clinical phenotype.
Unscheduled DNA synthesis studies were performed because of photosensitivity and poikilodermatous skin changes. Fibroblast cultures were grown from skin biopsy specimens of the two affected sisters, and the level of DNA repair was examined after UVC irradiation and incorporation of H3 thymidine. The investigations were normal.
DKC1 and TERC (dyskeratosis congenita) were screened for mutations by denaturing HPLC analysis. KIND1 (Kindler syndrome), and RECQL4 (RTS) were screened for mutations by direct sequencing of the entire coding region and splice sites. No mutations were found but the presence of several heterozygous polymorphisms in KIND1 and RECQL4 suggested that they were not candidates, given the consanguineous pedigree.
Polymorphonuclear studies
Human neutrophils were isolated from 16 ml of heparinized venous blood withdrawn from the patient, and normal volunteers as described [Böyum, 1968], after having obtained their informed consent.
The phagocytic capacity and the reactive oxygen species (ROS) production of the patient neutrophils, and healthy donors were estimated with the NBT reduction and phagocytosis slide test after activation with opsonized latex beads. The NADPH oxidase activity of intact neutrophils was assessed by measuring the rate of superoxide-sensitive cytochrome c reduction at 550 nm in the presence of superoxide dismutase (SOD) after phorbol myristate acetate (PMA) (20 ng/ml) or N-Formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) (0.1 µM) [Stasia et al., 2003]. The external peroxide hydrogen production of neutrophils activated with PMA (10 ng/ml), fMLP/platelet-activating factor (PAF) (1.25 µM/1.25µM) or opsonized zymosan (ZOPS) (1 mg/ml) was measured by the Amplex Red fluorescence method according to the manufacturer's instructions (Molecular Probes, Leiden, The Netherlands).
The results are presented in Table III. In the patient, at the age of 14 years, neutrophil function tests showed a normal phagocytosis of opsonized latex beads. ROS production in neutrophils activated by opsonized latex beads and measured by the NBT reduction test, was slightly reduced in the patient neutrophils (74% of the patient neutrophils reducing intracellular NBT vs. 86 ± 6% in control neutrophils). The extra cellular superoxide or peroxide production was evaluated by the superoxide dismutase-inhibitable cytochrome c reduction assay and the Amplex red fluorescence method. With both methods, ROS production was clearly reduced when the patient neutrophils were activated with soluble stimuli (PMA or fMLF or fMLF/PAF) that are known to produce extra cellular ROS in the majority, compared to the ZOPS activation, a particulate stimulus that produces higher intracellular ROS amounts.
| Tests | Patient | Control values (n = 13) |
|---|---|---|
| Phagocytosis of opsonized latex beads (% PMN)a | 98 | 95 ± 4 |
| NBT reduction (% PMN)b | 74 | 86 ± 6 |
| Respiratory burst | ||
| Superoxide generation (nmol red. cyt. c/106 PMN/min) | ||
| PMA (20 ng/ml) | 8.4–7.6c | 12.3 ± 3.5 |
| fMLF (0.1 µM) | 1.5 | 8.6 ± 3.9 |
| Peroxide production (µmol H2O2/5 × 104 PMN/10 min) | ||
| PMA (10 ng/ml) | 2.3–2.4c | 7.7 ± 2.0 |
| fMLF/PAF (0.1 µM/0.1 µM) | 4.6–4.2c | 5.7 ± 2.1 |
| ZOPS (1 mg/ml) | 3.6–3.9c | 2.9 ± 0.9 |
- NBT, nitroblue tetrazolium; PMA, phorbol myristate acetate; fMLF, N-formyl-methionyl-leucyl-phenylalanine; PMN, polymorphonuclear neutrophils, cyt. c, cytochrome c; ZOPS, opsonized zymosan.
- NADPH oxidase activity of the patient neutrophils was determined by NBT reduction slide test, superoxide dismutase-inhibitable cytochrome c reduction assay and the fluorescence method using Amplex red, as described in Materials and Methods. Control data represent the mean ± SD. The phagocytosis capacity of neutrophils was evaluated during the NBT reduction test after activation with opsonized latex beads.
- a Percentage of neutrophils that reduce the NBT.
- b Percentage of neutrophils that phagocytose opsonized latex beads.
- c Duplicate assays.
DISCUSSION
We report on a familial observation of poikiloderma with specific dermatological features, associated with dysmyelopoiesis and severe pulmonary infections with altered antioxidant responses. We have summarized on Table IV the major findings of the kindred against those of other well defined hereditary poikilodermas.
| RTS OMIM #268400 | Bloom syndrome OMIM #210900 | Xeroderma pigmentosum OMIM #278700 and others | Kindler syndrome OMIM #173650 | DKC OMIM # 127550, # 305000, 224230 | CLERICUZIO Type PKD with neutropenia OMIM #604173 | Reported kindred | |
|---|---|---|---|---|---|---|---|
| SKIN | |||||||
| Onset of PKD | Early | Early | Early | Progressive | Late | Early | Early |
| Photosensitivity | +/− | ++ | +++ | + | − | − | ++ |
| Blistering | +/− | − | − | ++ | − | − | ++ |
| Distribution | Face, limb. | Face | Photodistributed | Acral, diffuse | Face, trunk | Acral | Acral |
| PPK | +/− | − | − | + | +/− | + | + |
| Hair | + | − | − | − | + | − | − |
| Nails | − | − | − | +/− | + | + | + |
| Mucous Membranes | − | +/− | ++ | − | − | ||
| NON SKIN | |||||||
| Features | + | + | − | − | − | − | − |
| Development | GD, MR | GD++, MR | MR +/− | − | − | − | Mild MR, GD |
| Eye | +/− | − | + | − | + | − | + |
| Teeth | + | + | − | + | + | − | + |
| Skeletal | + | + | − | + | +/− | − | + |
| Others | ++, Endocrine.visceral, | Resp, GU, endocr, immuno deficiency | Neurologic | Webbing, Pseudo ainhum toes | GI, GU | Neutropenia, | Neutropenia, resp, ROM, |
| Complications | Neoplasia (BCC, SCC, osteogenic sarcoma) | Leukemia lymphoma | Early BCC, SCC, melanoma | SCC | Aplastic anemia SCC infections | Bronchopulmonary infections ETN | Bronchopulmonary, eye, ETN and skin infections |
| Inheritance | AR | AR | AR | AR | X-linked, AD AR | AR | AR |
| Cellular, molecular findings | RECQL4 8q24.3 | High SCE rate high chromosome breakage RECQL3 15q.26.1 | Defective DNA repair after UV damage, mutations in NER genes | Mutation in Kindlin 1 gene 20p12.3 | Dyskerin 1 gene (Xq28) TERC gene (3q21–3q28) | Neutrophil activation defects exclusion of the RECQL4 gene mutations. | Neutrophil activation defects, exclusion of other gene defects |
- AR, autosomal recessive; AD, autosomal dominant; BCC, basal cell carcinoma; GI, gastro intestinal; GR, growth retardation; GU, genitourinary; MR, mental retardation; PKD, poikiloderma; PPK, palmoplantar keratoderma; SCC, squamous cell carcinoma; SCE, sister chromatid exchange; GD, growth delay; NER, nucleotide excision repair; ETN, erythema toxicum neonatorum.
The pedigree of this early onset disorder strongly favors an autosomal recessive inherited condition. Skin manifestations develop in the first year of life on the face and extremities with an ichthyosiform appearance, associated with mild erythema, which subsequently evolve into a reticulated pattern of poikiloderma diffusing to the rest of the integument including a mild palmoplantar keratoderma (PPK). Photosensitivity and blistering with atrophic scars (dorsum of hands and fingers) are other dermatological characteristic features. There is no major sign of dysplasia of the ectodermal structures except pachyonychia affecting only the big toenails, carious dentition without frank enamel dysplasia, that is, abnormal shaped teeth, and an absence of hair and sweat anomalies. Compared to dyskeratosis congenita [Sirinavin and Trowbridge, 1975], mucous membranes are unaffected, although an obstruction of lachrymal ducts was found in the three children. The prominent dermatological presentation is not associated with osseous or soft tissue malformations or dysmorphic features. A mild growth delay is observed mainly by the 3rd–4th year but this could be attributed to the frequent infectious complications. A moderate developmental delay is also present.
The most prominent extracutaneous feature is the severe pulmonary disease, including recurrent bronchopneumonitis evolving into chronic pulmonary disease. Although sweat chloride tests were positive, a diagnosis of cystic fibrosis was ruled out by molecular analyses. Indeed false positives may occur particularly in chronic viral bronchopneumonitis as well as in chronic skin disorders such as atopic dermatitis [Brand et al., 1996]. Infections of the respiratory tract associated to poikiloderma have been reported in the RTS [Snels et al., 1998] associated with a low total number of T lymphocytes with a mildly depressed delayed immune reactivity. In our patients, the recurrent respiratory infectious episodes are apparently the consequence of chronic neutropenia, whereas the polyclonal hypergammaglobulinaemia (IgG, IgA) associated with increased C3 complement fraction is probably secondary to these episodes. To better characterize a possible immune deficiency associated with this particular type of poikiloderma, we looked for HPV-associated genotypes and particularly the oncogenic HPV 5 associated with epidermodysplasia verruciformis [Jablonska et al., 1972; Orth et al., 1979]. This resulted in the identification of an HPV genotype associated with common warts. Leukoneutropenia developed between 6 and 14 months of age and was constant with no correction during infectious periods. A variable asymptomatic thrombocytopenia was associated permanently or transiently in the first months of life. A moderate anemia also occurred and was probably not syndromic but rather related to iron deficiency and chronic inflammation. Overall, in view of the combination of the peripheral blood cell counts and abnormal bone marrow smear findings, hematological abnormalities appear to be related to myelodysplasia, which is known to be associated with a risk of leukemic transformation [Nishino and Chang, 2005]. In addition to neutropenia, neutrophil dysfunction consisting of a defect in the oxidative metabolism was observed in our patients and may be contributing to the susceptibility to infections. Oxidative burst is normally induced in peripheral blood phagocytes by the activation of NADPH oxidase. The NADPH-oxidase enzyme system is responsible for the generation of reactive oxygene species by forming a small transmembrane electron-transport system that results in the oxidation of NADPH on the cytoplasmic surface with the generation of superoxides on the outer surface of the cell membrane. This in turn becomes the inner surface of the phagosome when invagination occurs during phagocytosis. Generated superoxide and related toxic oxygen metabolites have a bactericidal activity and thus play an essential role in the host defence against microbial pathogens. In our patients as observed in patients with chronic granulomatous disease (CGD), production of superoxide is decreased [Finn et al., 1990; Rae et al., 1998]. Patients with CGD usually present early in life with multiple pyogenic infections with several degrees of severity as observed in our patients. Analysis of the different oxidase components has helped to define respiratory burst organization and assembly during phagocyte activation. Defects in different genes encoding protein components of this oxidase complex have been identified in CGD [Dinauer, 2005].
Several differential diagnoses were considered but differed from our cases as shown in Table IV.
In 1991 a new syndrome of poikiloderma associated with neutropenia was reported by Clericuzio among Navajo Indians [Clericuzio et al., 1991; Erickson, 1999]. The presentation of Clericuzio-type poikiloderma with neutropenia is characterized by an early onset of erythematous edematous papular lesions on the limbs and the face, which develop into telangiectatic lesions and poikiloderma. An involvement of the trunk may be seen on long term follow up; there is no clearly reported photosensitivity. A mild generalized hyperkeratosis, PPK, and pronounced thickening of toenails are other cutaneous findings, but no mucosal or ectodermal dysplastic features of hair or teeth were reported. The main extra cutaneous finding consists of a chronic leuconeutropenia found to be secondary to myelodysplasia. In addition, a polymorphonuclear activation defect was found and related to decreased bactericidal activity [Clericuzio et al., 1991; Erickson, 1999; Van Hove et al., 2005]; this functional defect was comparable to but less pronounced than that seen in CGD (OMIM306400). However serious lung diseases were reported, fatal in one case, and comparable to pulmonary lesions of CGD [Van Hove et al., 2005]. Linkage studies excluded the RECQL4 gene [Wang et al., 2003a; Van Hove et al., 2005], allowing the authors to make the distinction between the Clericuzio-type poikiloderma with neutropenia and RTS. Moreover, given this genetic distinction some authors [Van Hove et al., 2005] assumed that similar patients reported with the RTS poikiloderma associated to myelodysplasia and neutropenia [Bellinati-Pires et al., 1989; Rizzari et al., 1996; Narayan et al., 2001; Pianigiani et al., 2001], are more likely to be reclassified in this new group because of lack of extracutaneous features of RTS. Our observations in this family share many features with this newly described syndrome. Even though we report on some distinctive features in our cases, such as photosensitivity and blistering, ophthalmic and dental findings, the anomalies in polymorphonuclear oxidative burst provide evidence that the disorder reported in this family belongs to the Clericuzio type of poikiloderma with neutropenia. This oxidative burst anomaly suggests new therapeutic approaches to treatment for this disorder. In CGD patients, IFN-γ has been found to improve phagocyte NADPH oxidase activity [Dinauer, 2005]. IFN-γ could also be interesting in treatment of patients with Clericuzio type of poikiloderma with neutropenia. Moreover in CGD, the identification of molecular defects of components of NADPH oxidase responsible for the symptoms has helped to develop new therapeutic strategy.Gene transfer into hematopoietic stem cells has been successfully used to correct immunodeficiencies and has been successfully developed in CGD [Ott et al., 2007]. The understanding of the precise defect responsible for the Clericuzio type of poikiloderma with neutropenia will help to develop more targeted therapies.
Acknowledgements
We would like to thank A. Sarasin, J. Kolhase, I. Dokal, A. Iron, L. Lu, G. Meneguzzi, and M. Favre for their help with cellular and molecular analyses. Supported by the GENESKIN coordination action EU FP6 LSHB-CT-2005-512117.




