Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade†‡
How to cite this article: Cesarini L, Alfieri P, Pantaleoni F, Vasta I, Cerutti M, Petrangeli V, Mariotti P, Leoni C, Ricci D, Vicari S, Selicorni A, Tartaglia M, Mercuri E, Zampino G. 2009. Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. Am J Med Genet Part A 149A:140–146.
Laura Cesarini and Paolo Alfieri contributed equally to the work.
Abstract
Mutations in genes coding for transducers participating in the RAS/MAPK pathway have been identified as the molecular cause underlying a group of clinically related developmental disorders with cognitive deficits of variable severity. To determine the spectrum of cognitive defects associated with dysregulation of this signal cascade, we studied the profile of cognitive abilities in patients with mutations affecting the PTPN11, SOS1, HRAS, KRAS, BRAF, RAF1, and MEK1 genes and phenotype–genotype correlations. Our findings support the observation that heterogeneity in cognitive abilities can be at least partially ascribed to the individual affected genes and type of mutation involved. While mutations affecting transducers upstream of RAS were less frequently associated with mental retardation, mutations in downstream components of the pathway were generally associated with a more severe cognitive impairment. Among patients with a heterozygous PTPN11 mutation, the T468M substitution was associated with a mean IQ significantly higher compared to that of individuals carrying the N308D change. Our study provides insights on the range of cognitive abilities in patients with gene mutations causing dysregulation of RAS signaling suggesting that the presence and severity of cognitive involvement can be predicted in part by the gene involved. © 2009 Wiley-Liss, Inc.
INTRODUCTION
In the last few years, mutations in genes encoding for transducers participating in the RAS/MAPK signaling pathway have been identified as the molecular cause underlying a group of clinically related developmental disorders with features including reduced linear growth, dysmorphic facial features, congenital heart defects, skeletal and ectodermal anomalies and a variable degree of cognitive deficits [Tartaglia and Gelb, 2005; Gelb and Tartaglia, 2006; Kratz et al., 2007; Schubbert et al., 2007]. Noonan syndrome (OMIM 163950), which is the most common condition among these Mendelian conditions, is caused by heterozygous gain-of-function mutations in the PTPN11, SOS1, KRAS, and RAF1 genes in approximately 65% of affected individuals [Tartaglia et al., 2001, 2007; Carta et al., 2006; Schubbert et al., 2006; Pandit et al., 2007; Razzaque et al., 2007; Roberts et al., 2007; Zenker et al., 2007a,b]. PTPN11 and RAF1 mutations also account for the vast majority of LEOPARD syndrome (OMIM 151100) [Digilio et al., 2002; Legius et al., 2002; Pandit et al., 2007]. Defects in either KRAS, BRAF, MEK1, or MEK2, and various missense changes in HRAS have been observed in approximately 50–80% of patients with cardiofaciocutaneous (CFC) syndrome (OMIM 115150) [Niihori et al., 2006; Rodriguez-Viciana et al., 2006; Narumi et al., 2007; Nava et al., 2007; Schulz et al., 2008], and in Costello syndrome (OMIM 218040) [Aoki et al., 2005; Narumi et al., 2007; Nava et al., 2007; Rauen, 2007; Zampino et al., 2007; Schulz et al., 2008], respectively (Fig. 1). The phenotypic overlap occurring among these disorders justifies their grouping within a single “neurocardiofaciocutaneous” syndrome family [Bentires-Alj et al., 2006], but the substantial variation observed among and within each of these conditions points out the specific role of individual mutations in perturbing development and cognitive processes [Kratz et al., 2007; Schubbert et al., 2007].
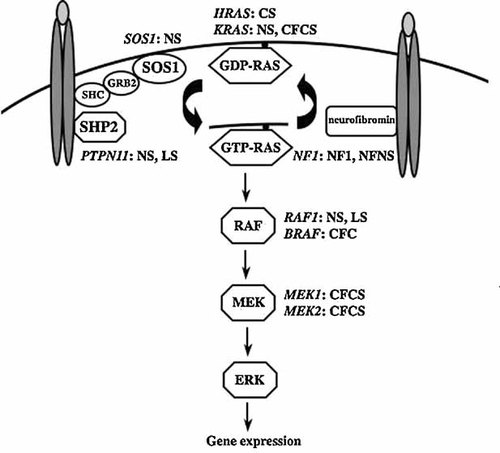
Dysregulated RAS/MAPK signaling in Noonan syndrome (NS), LEOPARD syndrome (LS), neurofibromatosis type 1 (NF1), neurofibromatosis-Noonan syndrome (NFNS), cardiofaciocutaneous syndrome (CFCS) and Costello syndrome (CS). Genes encoding for transducers known to be mutated in these Mendelian traits are shown.
Consistent with the experimental data generated by murine models indicating a key-role for RAS signaling in memory, learning [Sweatt, 2001, 2004; Sweatt and Weeber, 2003; Sweatt et al., 2003], and, more generally, cognitive abilities [Atkins et al., 1998; Mazzucchelli and Brambilla, 2000; Sweatt, 2001; Weeber and Sweatt, 2002; Thomas and Huganir, 2004], it is not surprising that mental retardation frequently occurs in patients with disorders of the RAS/MAPK signaling cascade. The Neurocardiofaciocutaneous-causing gene mutations ultimately promote dysregulation of RAS signaling, but their perturbing consequences on signal flow, and developmental processes and cognition is expected to differ considerably, from both a qualitative and quantitative viewpoint. Several factors are predicted to explain such a differential effect, including the signaling hierarchy of individual transducers (signal amplification and branching, and negative feedback control), cell context relevance of each pathway component, as well as a differential effect of individual mutations in perturbing the biochemical properties and function of the encoded protein [Kratz et al., 2007]. Consistently, genotype–phenotype correlations support this view, documenting that a considerable portion of phenotypic heterogeneity occurring in each of these disorders can be ascribed to genetic heterogeneity [Tartaglia et al., 2002, 2007; Zenker et al., 2004, 2007b; Narumi et al., 2007; Nava et al., 2007; Pandit et al., 2007; Schulz et al., 2008].
In this paper we assessed cognitive abilities in a cohort of patients with Noonan, LEOPARD, Costello, and CFC syndromes in whom germline PTPN11, SOS1, KRAS, HRAS, BRAF, RAF1, MEK1, and MEK2 gene had been identified in order to assess whether spectrum of cognitive defects in these disorders can be at least partially explained taking into account individual affected genes and type of mutation.
SUBJECTS AND METHODS
Patient Cohort
The subjects included in the study were followed on a regular basis at the Department of Pediatrics of the Catholic University (Rome, Italy) and Clinica De Marchi (Milan, Italy), and are part of a prospective study aimed to the clinical and molecular characterization of these disorders [Tartaglia et al., 2001, 2007; Pandit et al., 2007; Zampino et al., 2007]. Subjects were examined by experienced clinicians, and diagnoses were based on clinical features previously reported [Voron et al., 1976; Van der Burgt et al., 1994; Hennekam, 2003; Roberts et al., 2006; Gripp et al., 2007]. Within groups, clinical features satisfied diagnostic criteria for each condition. Based on the clinical diagnosis, patients were screened for mutations in the entire coding sequence of the PTPN11, KRAS, SOS1, BRAF, and RAF1 (Noonan/LEOPARD syndrome), HRAS (Costello syndrome), or KRAS, BRAF, MEK1, and MEK2 (CFC syndrome) genes [Pandit et al., 2007; Tartaglia et al., 2007; Zampino et al., 2007]. Primer sequences and protocols utilized for molecular characterization of patients are available on request. In this study, only patients for whom a disease-causative mutation was found were included (Table I).
| Clinical diagnosis preceding genetic analysis | Affected gene | Gender | Age range (years) | Mean age (years) |
|---|---|---|---|---|
| Noonan/LEOPARD syndrome | PTPN11 | 10M/9F | 0.7–15.4 | 9.0 |
| SOS1 | 3M/3F | 3.2–35.6 | 15.7 | |
| RAF1 | 1M/2F | 7.4–15.5 | 10.4 | |
| KRAS | 1M | 3.6 | 3.6 | |
| Costello syndrome | HRAS | 4M/5F | 0.8–28.0 | 11.5 |
| CFC syndrome | BRAF | 1M/9F | 1.5–21.1 | 8.0 |
| MEK1 | 1F | 7.9 | 7.9 |
- M, male; F, female.
Assessment of Cognitive Abilities
Cognitive development was assessed using the Wechsler Preschool and Primary Scale of Intelligence—Revised (WPPSI) or the Wechsler intelligence Scale for Children-Revised (WISC-R) and the Wechsler Adult Intelligence Scale (WAIS) scales, depending on the age of the subject and if appropriate for the level of functioning. These tests, which are accepted worldwide for assessing the intellectual ability of patients older than 4 years, consist of ten subtests that allow evaluation of verbal and non-verbal (performance) abilities. The results of the tests are expressed as a global intelligence quotient (GIQ), a verbal intelligence quotient (VIQ) and a performance intelligence quotient (PIQ), based on age specific normative data. As we were interested in a global measure of IQ including both verbal and non-verbal abilities and in possible discrepancies between them, we used the Wechsler scales rather than the Leiter-r scale. The latter is known to be more precise at the lower end of the spectrum but does not include any item to investigate verbal abilities. The Wechsler scales used in this study were the most recent validated and standardized versions available in Italy at the time the enrollment of patients started.
Mental development was classified according to the classification reported in the Diagnostic and Statistical Manual of Mental Disorders 1994 (DSM-IV). Children younger than 4 years were assessed using Griffiths Mental Developmental Scales [Griffiths and Huntley, 1996] that include different subscales (locomotion, language, personal–social, eye–hand coordination, performance, and practical reasoning) and provide scores for each subscale and a global score (developmental quotient, DQ). All evaluations were performed by two examiners (LC and PA) blind to the genetic data.
Statistical Analysis
Descriptive analyses were conducted to examine the distribution of variables of interests. VIQ, PIQ and GIQ distributions were compared between groups by means of the Student's t-test. All analyses were carried out with the STATA statistical package release 7.0 (Stata Corporation, College Station, TX).
RESULTS
The cohort included 49 patients (20 males and 29 females) heterozygous for a missense mutation in PTPN11, SOS1, HRAS, KRAS, BRAF, RAF1, or MEK1 with a clinical diagnosis of Noonan/LEOPARD syndrome (N = 29), Costello syndrome (N = 9), or CFC syndrome (N = 11), and age ranging between 8 months and 35 years (Table I). Five patients with CFC syndrome were not assessed because of severe cognitive and behavioral problems. Of the remaining 44 patients, 13 were assessed using the Griffiths' Scales, 3 on the WPPSI, 25 on the WISC-R and 3 on the WAIS. Overall distributions of cognitive abilities within individual disorders and groups of subjects classified according to the molecular data are shown in Supplementary Table II.
Three of the 19 subjects heterozygous for a missense mutation in PTPN11 were assessed on the Griffiths' Scales, and showed a mean DQ of 84.3. Sixteen patients were above the age of 4 years, and their global IQ ranged between 57 and 115 with a mean of 90.7 (Fig. 2). Mean verbal IQ was 93.1 and mean performance IQ was 90.3. Five subjects were heterozygous for the recurrent T468M amino acid substitution and showed a GIQ within normal limits (higher than 96 in all cases) as well as significantly higher VIQ, PIQ and GIQ compared to the other subjects harboring different mutations in the same gene (VIQ: 100.0 ± 3.1 vs. 89.9 ± 11.8, P = 0.09; PIQ: 106.4 ± 15.0 vs. 83.0 ± 14.3, P = 0.01; GIQ: 102.8 ± 8.4 vs. 85.2 ± 12.7, P = 0.01). Patients with the N308D change, which represent the most common mutation occurring among subjects with Noonan syndrome, exhibited significantly lower scores (VIQ: 79.3 ± 13.0 vs. 97.7 ± 4.8, P = 0.001; PIQ: 72.8 ± 14.5 vs. 96.2 ± 15.3, P = 0.01; GIQ: 74.0 ± 13.3 vs. 96.3 ± 9.3, P = 0.002). The profiles of the mean scores of the Wechsler subtests in the groups of subjects with N308D and T468M are shown in Figure 3.
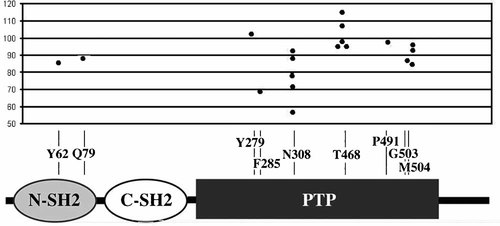
Distribution of individual global IQ scores referred to each mutation affecting the PTPN11 gene.
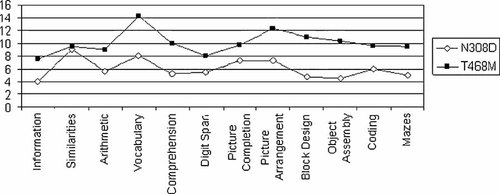
Profiles of the mean scores of the Wechsler subtests in subjects heterozygous for the N308D or T468M SHP2 amino acid change.
One of the six subjects carrying a heterozygous SOS1 mutation was assessed on the Griffiths' Scales and had a DQ of 104. Of the five patients above the age of 4 years, all but one had GIQ scores above 102 with a mean of 106.3. Of note, the only patient with low IQ had a history of neonatal encephalopathy and had epilepsy. Comparison of VIQ, PIQ, and GIQ distributions between these subjects and those heterozygous for a mutated PTPN11 allele did not reveal any statistically significant difference (VIQ: 96.2 ± 24.8 vs. 93.1 ± 10.9, P = 0.686; PIQ: 92.8 ± 28.4 vs. 90.3 ± 18.0, P = 0.816; GIQ: 93.8 ± 28.1 vs. 90.7 ± 14.1, P = 0.739). In contrast, subjects with a germline HRAS or KRAS gene mutation were characterized by a severe cognitive impairment. Specifically, among the nine individuals heterozygous for the G12S amino acid change of HRAS, 4 were assessed on the Griffiths' Scales and had a mean DQ of 53.2. Five patients were above the age of 4 years, and their global IQ ranged between 41 and 55 with a mean of 48.2. Consistently, they exhibited significantly lower VIQ, PIQ, and GIQ scores (VIQ: 55.2 ± 7.38; PIQ: 47.8 ± 8.2; GIQ: 93.8 ± 28.1) compared to those of subjects with mutations in PTPN11 or SOS1 (P < 0.01 in all comparisons). Similarly, the single subject with a heterozygous KRAS change was found to have a DQ of 56.
Among the 14 subjects with mutations in genes coding for RAS effectors or downstream transducers, 10 were mutated in the BRAF gene. Four of them were assessed on the Griffiths' Scales, and had a mean DQ of 47.5. Of the six patients above the age of 4 years, five were untestable because of the profound impairment and only one had a GIQ within the normal range. Two of the five untestable patients had refractory epilepsy. In contrast, patients with a mutation affecting the RAF1 gene, which encode a serine/threonine protein kinase structurally and functionally related to BRAF, were documented to have less severe cognitive deficit, showing a GIQ that ranged between 70 and 104 with a mean of 81.3. Finally, the single patient with a heterozygous MEK1 change had a GIQ lower than 40.
The distribution of GIQ scores in the various groups subdivided according to their genotype is shown in Figure 4. No obvious discrepancy between VIQ and PIQ in all subgroups examined was observed (P > .05).
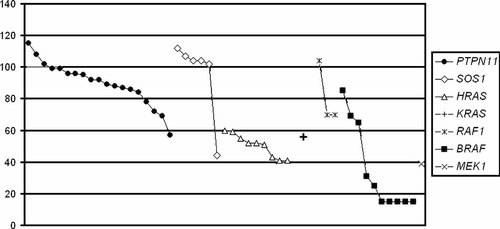
Distribution of individual global IQ scores referred to each affected gene.
DISCUSSION
The aim of the study was to assess cognitive abilities of subjects with disorders caused by heterozygous germline mutations in genes coding for transducers in RAS/MAPK signaling. The relevance of dysregulated RAS signaling in human cognition has previously been reported in patients with neurofibromatosis type 1, who exhibit heterozygous loss of function mutations or deletions of NF1, the gene encoding for the RAS-specific GAP neurofibromin [Lee and Stephenson, 2007]. These patients usually have a slight downward shift in IQ compared to the general population, with mean scores clustering around the low average to average range [Levine et al., 2006]. The crucial role of the RAS/MAPK cascade in cognition has also been demonstrated in animal models, particularly in processes controlling neuronal plasticity, memory and learning [Sweatt and Weeber, 2003; Sweatt et al., 2003]. RAS signaling has been documented to be implicated in the control of cell division and differentiation during nervous system development, dendritic organization in differentiated neurons as well as to promote synaptic connectivity of cortical neurons, both at the structural and functional level [Kim et al., 2004]. The activation of the RAS/MAPK pathway has also been observed to be involved in triggering long term synaptic changes in the mammalian central nervous system, [Sweatt et al., 2003; Sweatt, 2004; Thomas and Huganir, 2004] particularly in the hippocampal area CA1 and amygdale, dentate gyrus, and other areas involved in learning and memory [Selcher et al., 2003].
Although there is substantial clinical evidence for cognitive impairment in disorders caused by mutations in genes coding for proteins downstream the RAS/MAPK cascade, including Coffin Lowry syndrome [Hanauer and Young, 2002], Rubenstein-Taybi syndrome [Hennekam, 2006], and Fragile X syndromes [Kim et al., 2008], little has been reported about cognitive abilities in patients with mutations in upstream components of this signaling pathway, such as those recently identified in patients with neurocardiofaciocutaneous syndrome. Until recently, those patients were classified on the basis of their clinical features and signs, with a reported wide variability in cognitive abilities in each syndrome [Voron et al., 1976; Delrue et al., 2003; Lee et al., 2005; Roberts et al., 2007; Van der Burgt, 2007]. A precise evaluation of the range of cognitive abilities was further complicated by the heterogeneity in classification and inclusion criteria for each syndrome, in absence of any genetic or molecular information. Only a few recent studies have evaluated cognitive abilities in subjects genetically characterized but data have so far been limited to KRAS, HRAS, BRAF, and MEK1 [Schubbert et al., 2006; Axelrad et al., 2007; Nava et al., 2007; Yoon et al., 2007; Zenker et al., 2007b]. To provide more information, the present study explores cognitive abilities associated with PTPN11, SOS1, and RAF1 mutations. We also provide additional data on cognitive abilities of subjects with KRAS, HRAS, BRAF, and MEK1 defects. Together with previously published data, our findings allow a more general picture suggesting that the level of cognitive abilities is correlated to the individual gene involved. Specifically, mutations in genes that encode for transducers with function upstream of RAS appear to be less frequently associated with mental retardation. We observed that only three of the 25 patients (12%) with mutations in PTPN11 or SOS1 had low IQ values. A recent study reported a mean IQ of 90 in patients with Noonan syndrome due to mutations in PTPN11 or SOS1, even though no details on individual IQs and their distribution according to the genotype were available [Verhoeven et al., 2008]. Patients heterozygous for a RAF1 mutation appear to have borderline or normal results.
Mutations affecting HRAS or genes coding for transducers functioning downstream along the cascade were, in contrast, more often associated with low IQ scores. Only two of the24 (8%) patients heterozygous for a HRAS, KRAS, RAF1, BRAF, or MEK1 mutation had an IQ within normal limits. These results are consistent with recently published data documenting normal IQs in only two out of 49 (4%) patients with mutations affecting the HRAS, KRAS, BRAF, or MEK1 gene, with four additional patients with borderline results (Supplementary Table I). Of note, the present study also indicates that within this group of patients, a certain degree of genotype–phenotype correlation can be found. More specifically, while BRAF and MEK1 mutations are more often associated with severe mental retardation, HRAS defects are generally associated with less severe cognitive impairment. Although the size of our cohort is not sufficiently large to allow a meaningful statistical analysis, there is a clear a trend of severity that is consistent with previous studies in patients carrying a KRAS, HRAS, BRAF, or MEK1 mutated gene.
A certain degree of variability in cognitive abilities was also observed among patients with mutations affecting the same gene. While subjects with the G12S amino acid substitution in HRAS had similar cognitive profiles with a relatively narrow range of IQs, patients with a mutated PTPN11 allele had a wider spectrum of cognitive abilities, which appeared to correlate with the type of mutation. Within this group, patients heterozygous for the T468M substitution had IQs above 96, and a mean significantly higher compared to that of individuals carrying the N308D change. It should however be considered that a certain intra-group variability could also be attributed to clinical confounding factors, such as epilepsy, that occurred in the only patient with low IQ among the six with SOS1 mutation. The same SOS1 mutation has previously been documented in another patient who did not have epilepsy and a normal cognitive development [Zenker et al., 2007a].
CONCLUSION
We provide further details of the range of cognitive abilities in patients with gene mutations causing dysregulation of the RAS/MAPK cascade, and support the hypothesis that the observed heterogeneity in cognitive abilities can be at least partially ascribed to the individual affected genes and type of mutation involved. Although we assessed 49 patients, when we subdivided the cohort according to the type of mutation, the numbers in each group were relatively small to establish a precise genotype–phenotype correlation. Further studies in larger and more homogeneous cohorts using more specific assessments for memory and other aspects of learning that have been found to be affected in animal models [Sweatt et al., 2003; Sweatt, 2004] are required to achieve a more precise characterization of cognitive ability profiles associated with individual gene involved and type of molecular lesion.
Acknowledgements
We are indebted to the patients and families who participated in the study and the physicians who referred the subjects. This work was supported by the Telethon-Italy (GGP07115) and Programma di Collaborazione Italia-USA/malattie rare 2007 grants (to M.T.). A. S. and M.C. were supported by Mariani Foundation.




