Autosomal dominant gigantiform cementoma associated with bone fractures†
How to cite this article: Moshref M, Khojasteh A, Kazemi B, Roudsari MV, Varshowsaz M, Eslami B. 2008. Autosomal dominant gigantiform cementoma associated with bone fractures. Am J Med Genet Part A 146A:644–648.
Abstract
Here, we report a family with gigantiform cementomas, bone fractures, and autosomal dominant inheritance. Lesions are composed of benign, lobulated, calcified masses resembling cementum. Identification of a COL1A2 mutation in one patient was a polymorphism of no pathological significance. The subject of gigantiform cementomas and the associated bone disorder is both confusing and complex. Reported familial instances indicate genetic heterogeneity with (1) osteopenia and bone fractures, (2) one form of osteogenesis imperfecta, and (3) a polyostotic diaphyseal bone disorder. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Gigantiform cementoma constitutes one of the four subgroups of cementomas in the classification of the World Health organization [Pindborg et al., 1971]. They noted that “familial multiple cementomas” are lobuted masses of dense, highly calcified, cementum-like masses typically occurring in several parts of the jaws. These lesions may be inherited and in some instances can be associated with bone fractures. The subject, which is both complex and confusing, is analyzed further in the discussion. Here, we report on a family with gigantiform cementomas, bone fractures, and autosomal dominant inheritance.
CLINICAL REPORT
The pedigree (Fig. 1) shows affected individuals in four generations, indicating autosomal dominant inheritance.
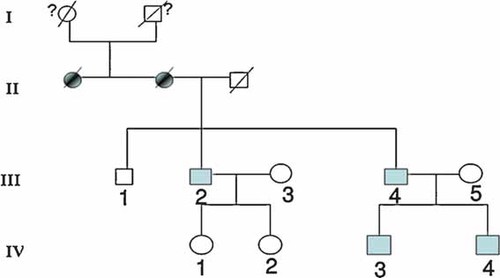
Pedigree showing autosomal dominant transmission. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 1 (III-4) was a 48-year-old Iranian man with maxillary expansion and chronic pus discharge from left side of the posterior maxilla. He became aware of bony expansion of both jaws at the age of 24 when a mandibular fracture occurred during extraction of a mandibular left first molar. Radiologic evaluation disclosed widespread, mixed radiolucent/radiopaque lesions in both jaws (Fig. 2A–C). The fracture was reduced and stabilized with wire, but osteomyelitis developed, and continual pus drainage was observed 9 months following surgery. Biopsy specimens from the maxilla and mandible consisted of cementum-like masses with rounded margins surrounded by fibrous connective tissue with a mononuclear cell inflammatory infiltrate and active fibrosis (Fig. 2D). At 42 years of age, the patient fractured his left proximal femur and since then, he has sustained long bone fractures caused by minor trauma five times within 2 years (Table I). Radiographs disclosed osteopenic bone and excess callus in the fracture areas (Fig. 3A,B). The patient walked with the stagger because of several fractures. A partial maxillectomy was performed, and oral rehabilitation was accomplished with a partial removable denture.
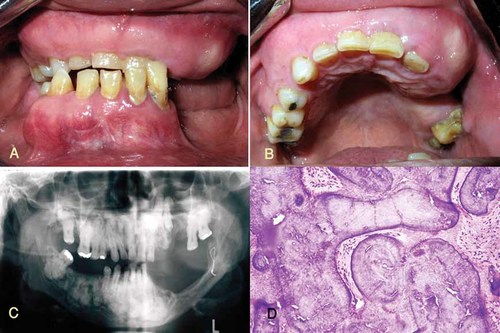
A,B: Intraoral appearance of the maxilla and mandible indicating buccal and palatal cortical expansion. C: Multiple radiopaque areas throughout the maxilla and mandible. D: Histopathology of benign fibro-cemento-osseous lesion (hematoxylin and eosin stain, magnification 400×). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Patient | Sex | Age of onset of mandibular lesion | Age of onset of long bone fractures | Site of fractures |
|---|---|---|---|---|
| 1 | M | 24 | 42 | Mandible, right humerus, both proximal femora, right tibia |
| 2 | M | 25 | 40 | Proximal humerus, tibia |
| 3 | M | 21 | 22 | Humerus, right proximal femur, left distal femur |
| 4 | M | 20 | — | — |
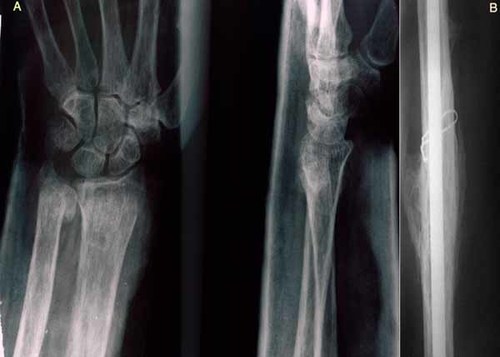
A,B: Osteopenic bone with excess callus formation after previous bone fracture.
Patient 2 (III-2) was the 51-year-old brother of Patient 1, who had experienced multiple fractures between the age of 35 and late adulthood (Table I). Radiographs of the jaws disclosed large, well-defined, lobular, mixed radiolucent/radiopaque masses surrounded by a narrow radiolucent zone (Fig. 4A–C). The exact time of onset of the lesion was not clear, but the patient had noted expansion of the mandibular cortical plate when he was 25 years old. Chronic osteomyelitis developed in the anterior mandible following the extraction of teeth. A large swelling of the bony cortical plate was found on the anterior ridge of the mandible. Histological examination of a biopsy specimen taken from this region demonstrated findings similar to those of his brother (Fig. 4D) (III-4). Alveoloplasty with curettage of the necrotic bone was carried out and the patient was referred for prosthodontic rehabilitation.
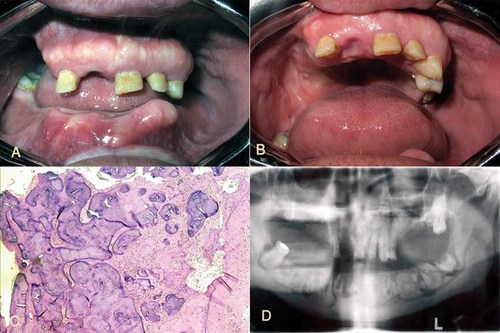
A,B: Intraoral appearance of the maxilla and mandible indicating buccal and palatal cortical expansion. C: Benign fibro- cemento-osseous lesion (hematoxylin and eosin stain, magnification 100×). D: Orthopantograph of the brother demonstrating lobular radiopaque areas throughout the body of the mandible. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 3 (IV-3) was the 25-year-old son of Patient 1. He had a history of three long bone fractures (Table I). Radiological evaluation disclosed extensive mixed radiopaque/radiolucent lesions in all four quadrants of the jaws (Fig. 5).
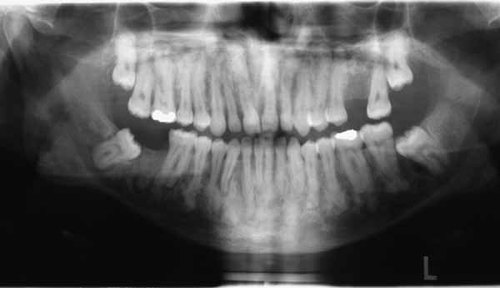
Initial lesion in the mandibular body of the first son.
Patient 4 (IV-4) was the 20-year-old youngest son of Patient III-4. When he was 17, radiological evaluation of the jaws did not show any bony lesions, but at 20, serial radiological investigations demonstrated radiolucent lesions at the apices of the maxillary teeth (Fig. 6). Buccal cortical expansion of the jaws had not appeared until this time. No long bone fractures had occurred by this age.
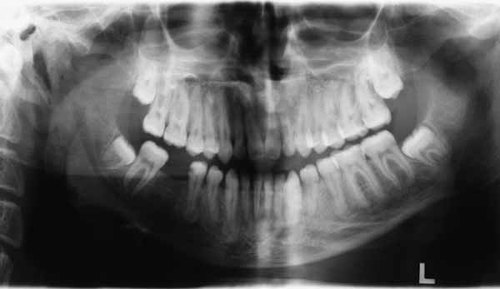
Serial radiological evaluation of the second son demonstrating radiopaque cemento-osseous lesions at the root apices of the maxillary and mandibular teeth.
MUTATIONAL ANALYSIS
Blood DNA from patients III-2 and III-4 was extracted [Sambrook and Russell, 2001] and analyzed by the PCR method [McPherson and Moller, 2000] with primers for COL1A2. In analyzing the gene sequence, a mutation was found in Patient III-4, TG replacing GT in nucleotides 14929 and 14930 of intron 26. This mutation in COL1A2 was a nonpathologic polymorphism.
DISCUSSION
We have documented a family with gigantiform cementomas of the jaws and bone fractures with autosomal dominant transmission. Histopathologically, the lesions are composed of benign, lobulated, calcified masses resembling cementum. They have been proposed to arise from the periodontal ligament [Waldron et al., 1999]. Gigantiform cementoma tends to arise at an early age and develops slowly [Young et al., 1989]. It has been stated to occur most commonly in middle-aged women of African or East-Asian origin [Thompson and Altini, 1989; Ackermann and Altini, 1992; Kramer et al., 1992; MacDonald-Jankowski, 1992; Kawai et al., 1999].
Some instances of gigantiform cementomas are described with observable expansion of the bony plates of the jaws, as in our patients and some patients of Young et al. 1989 (vide infra), but no expansion has been noted in other cases with three possibilities: (1) affected young patients have yet to develop the full lesions, (2) some articles do not mention expansion or lack of expansion, and (3) no expansion is present in some instances, even in adult patients, e.g., Levin et al. 1985.
The subject of gigantiform cementomas is both confusing and complex. Some instances are said to be sporadic [e.g., Norberg, 1930; Thompson and Altini, 1989; Ong and Siar, 1997], but most often, no family history was taken, and/or family members lacked physical examinations of the jaws, radiographs, and/or biopsies. Such information is said to be available in a review of cases in radiology at an Army medical center [Thompson and Altini, 1989], but this assurance is not convincing. Familial examples have been documented in some instance, exhibiting autosomal dominant transmission, or with few affected family members, consistent with autosomal dominant inheritance [Agazzi and Belloni, 1953; Akasaka et al., 1969; Cannon et al., 1980; Levin et al., 1985; Young et al., 1989; Coleman et al., 1996; Nishimura et al., 1996; Finical et al., 1999; Abdelsayed et al., 2001; Rossbach et al., 2005].
Second, in some instances, familial examples have bone fractures, and these have been variously termed “brittle bones” [Rossbach et al., 2005] “osteogenesis imperfecta,” [Levin et al., 1985] and “polyostotic diaphyseal bone disease” [Rossbach et al., 2005]. The various terms used for gigantiform cementomas are just as confusing, for example, “florid cemento-osseous dysplasia” [Coleman et al., 1996; Ong and Siar, 1997].
In the future, careful studies of affected families with clinical, radiographic, histopathologic, and molecular data need to be carried out. Nevertheless, some conclusions are warranted at the present time. Evidence to date strongly indicates genetic heterogeneity. The studies of Young et al. 1989, Rossbach et al. 2005, and Levin et al. 1985 summarized below demonstrate this.
The family reported by Young et al. 1989 with autosomal dominant transmission shows variability in expression. Some affected family members with gigantiform cementomas had bony expansion of the jaws and some did not. Some had bone fractures and some did not. Finally, some bone fractures occurred without jaw involvement. The autosomal dominant family reported by Rossbach et al. 2005 had gigantiform cementomas, polyostotic diaphyseal bone disorder, fractures, and osteosarcoma in one family member. Levin et al. 1985 in an article titled “Osteogenesis Imperfecta with Unusual Skeletal Lesions” reported two autosomal dominant families (and a third sporadic case) with bone fractures, multilocular radiolucent, radiopaque, or radiolucent-radiopaque lesions of the maxilla and mandible, coarseness of bone trabeculae, difffuse osteopenia, fractures, white sclerae, and normal teeth. They postulated that their families represented a newly delineated type of osteogenesis imperfecta. Jaw lesions did not exhibit expansion of bone, and no biopsies with histopathological study were possible.
Osteogenesis imperfecta per se is well-known to be genetically heterogeneous. Rauch and Glorieux 2004 reviewed the 7 known types of osteogenesis imperfecta of which the molecular basis of types I–IV are well known. Wenstrup 1997 indicated that over 200 mutations in COL1A1 and COL1A2 were known by 1997. The identification of a mutation in COL1A2 found in our patient III-4 but not in III-2 was a polymorphism of no pathologic significance. Gorlin et al. 2001 and Tinkle and Wenstrup 2005, besides reviewing osteogenesis imperfecta, have reviewed dozens of other genetic bone disorders, including those with autosomal dominant inheritance, in which bone fractures are also known to occur. Thus, there are many possibilities to consider.




