Severe neonatal non-dystrophic myotonia secondary to a novel mutation of the voltage-gated sodium channel (SCN4A) gene†
How to cite this article: Gay S, Dupuis D, Faivre L, Masurel-Paulet A, Labenne M, Colombani M, Soichot P, Huet F, Hainque B, Sternberg D, Fontaine B, Gouyon J-B, Thauvin-Robinet C. 2008. Severe neonatal non-dystrophic myotonia secondary to a novel mutation of the voltage-gated sodium channel (SCN4A) gene. Am J Med Genet Part A 146A:380–383.
Abstract
We report on a patient with a severe, rare neonatal form of non-dystrophic myotonia. The patient presented with facial dysmorphism, muscle hypertrophy, severe constipation, psychomotor delay, and frequent cold-induced episodes of myotonia and muscle weakness leading to severe hypoxia and loss of consciousness. Muscle biopsy was non-specific and electromyography revealed intense generalized myotonia. The myotonic episodes improved after introducing oral mexiletine and maintaining room temperature at 28°C. The patient died at 20 months of age following a bronchopulmonary infection. A previously undescribed de novo heterozygous c.3891C > A change, which predicts p.N1297K in the SCN4A gene. Mutations within the voltage-gated sodium channel alpha-subunit gene (SCN4A) have been described in association with several phenotypes including paramyotonia congenita, hyperkalemic or hypokalemic periodic paralysis, and potassium-aggravated myotonias. The cold-sensitive episodes of stiffness followed by weakness suggested the diagnosis of channelopathy in our patient. However, her neonatal onset, the triggering of severe episodes by exposure to modest decreases in temperature, involvement of respiratory muscles with prolonged apnea, early-onset muscle hypertrophy, psychomotor retardation, and fatal outcome are evocative of a distinct clinical subtype. Our observation expands the phenotypic spectrum of sodium channelopathies. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Non-dystrophic myotonias are skeletal muscular channelopathies, which have been associated with mutations within the sodium or chloride channels [Vicart et al., 2005]. Hyperkalemic (HYPP) or hypokalemic periodic paralyses, potassium-aggravated myotonias (myotonia fluctuans, myotonia permanens, and acetazolamide-responsive myotonia) and paramyotonia congenital (PC) are allelic diseases caused by mutations in the skeletal muscle voltage gated sodium channel gene (SCN4A). The SCN4A gene encodes the voltage-dependent Na+ channel in skeletal muscle, which is responsible for the rising phase of the action potential in the membranes of neurons and most electrically excitable cells [Catterall, 2000]. PC is usually characterized by dominant inheritance, myotonic symptoms not alleviated by repetition of movement (paradoxical myotonia), paretic symptoms that may follow myotonic episodes, a sensitivity to cold that aggravates or triggers myotonic as well as paretic symptoms, non-progressive nature; and a lack of marked atrophy or hypertrophy of muscles [McClatchey et al., 1992; Mohammadi et al., 2003; Bouhours et al., 2004]. The symptoms usually start in the first decade of life. To date, 15 mutations have been reported in the SCN4A gene in patients with PC, all resulting in gain-of-function defects [Vicart et al., 2005; Wu et al., 2005; Ferriby et al., 2006]. Therapy is based on local anaesthetics and class 1b antiarrhythmic agents such as lidocaine, mexiletine, and other lidocaine derivates [Jackson et al., 1994; Meola and Sansone, 2000]. Only one family with a PC-HYPP overlap syndrome has been described with a severe infantile presentation [Brancati et al., 2003]. Here, we report on a patient with severe fatal neonatal non-dystrophic myotonia secondary to a mutation in an intracytoplasmic loop of the SCN4A gene product.
CLINICAL REPORT
The patient was followed in our intensive care unit from birth to age 13 months for recurrent episodes of severe desaturation. She was the second child of non-consanguineous parents. The family history was unremarkable. Measurements at birth after 37 weeks of gestation were weight, 3,240 g (mean) length, 45 cm (<3rd centile), and OFC, 37 cm (90th centile). At birth, APGAR scores were 10/10. A few hours later, she presented tachypnea and abdominal distension, which required her admission in the intensive care unit. After recovery, apnea and hypoxia episodes recurred 48 h later. Facial features included high forehead, downslanting palpebral fissures, low-set ears, short neck, and high arched palate (Fig. 1A). No major malformations were found except congenital hip dislocation. Electrocardiogram and heart ultrasound were normal.
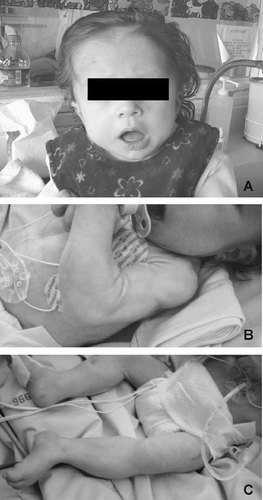
A: Facial dysmorphism with facial hypotonia, high forehead, low-set ears, short neck and high arched palate. The picture does not demonstrate the down slanting palpebral fissures since the eyes are obscured. B,C: Diffuse muscle hypertrophy, predominantly in the biceps brachii and triceps.
She subsequently presented with episodes of apnea from 1 to 10 times a day treated by manual ventilation. The typical prodrome included profuse sweating. Arm stiffness and loss of consciousness were present during all attacks, followed by severe desaturation and cyanosis. They ended with general muscle weakness and heavy inspiration which provided a rapid return to normal vital signs. Between such episodes, serum potassium levels were normal.
At 4 months of age, she had severe failure to thrive with height (56 cm) and weight (3,980 g) below—3 SD despite gastrostomy for enteral feeding with major caloric supplementation and Nissen fundoplication at 2 months of age for severe gastroesophageal reflux. Constipation responded to chronic laxative treatment.
Neurological examination showed hypotonia with peripheral hypertonia. Tendon reflexes were present but polykinetic. There was neither myotonia at percussion nor palpebral myotonia. Facial movements were poor. Psychomotor delay was severe (development quotient with Brunet-Lezine scale = 38; normal: 100).
At age 6 months muscle hypertrophy appeared predominantly in the biceps and triceps (Fig. 1B,C). Triceps hypertrophy was firstly noted at 4.5 months of age. At 9 months of age, the child had persistent desaturation, bradycardia, and generalized stiffness. Treatment with carbamazepine (20 mg/kg/day) was attempted but stopped 1-week later because of the absence of improvement of the symptoms. A cooling test was performed by immersion of the lower limbs in water at 25°C, which immediately induced an attack of fainting and muscle weakness. CPK level was 142 UI/l (normal: 30–135). Oral therapy with mexiletine (15 mg/kg/day) was introduced. Maintenance of a therapeutic blood level of mexiletine (0.5 mg/L; N:0.5–2 mg/L) and cutaneous temperature of around 38°C resulted in a significant decrease in myotonia. She was discharged at 13 months of age.
At 18 months of age, she presented with persistent growth and weight retardation. Length was 67.5 cm (−4 SD) and weight was 7.800 g (−2.5 SD). She was able to sit and she caught objects with difficulty but hypotonia with peripheral hypertonia and muscle hypertrophy were persistent. Mexiletine doses were increased to 17 mg/kg/day to be effective. Some attacks of myotonia reappeared. The serum level of mexiletine was in the therapeutic range for the treatment of myotonia in adults. She was readmitted at 20 months of age and died secondary to prolonged respiratory arrest associated with a bronchopulmonary infection.
Etiological investigations including serum potassium levels, standard and high resolution chromosomal analyses (700 band resolution according to ISCN standards), subtelomeric fluorescent in situ hybridization (FISH) studies, plasma amino acid and urinary organic acid chromatographies were normal. Muscle biopsy was non-specific, without vacuoles, fibrosis or inflammatory cells. Several fibers contained excessive mitochondria without aggregates. Electromyography with concentric needle electrodes revealed intense generalized electric myotonia. Neither voluntary nor automatic muscle activity was possible due to the young age of the patient. Since the patient could not exercise, the cubital nerve was stimulated before and after faradization (40 stimulations at 20 Hz). The motor potentials of the abductor digiti quinti did not decline significantly in amplitude, which supported the diagnosis of non-dystrophic myotonia. Sensory and motor nerve conductions were normal for the age. DM1 locus analysis, perlecan gene sequencing on fibroblasts and GLRA1 gene sequencing on blood DNA samples were normal. Finally, SCN4A gene direct sequencing showed the presence of a heterozygous c.3891C > A mutation, which predicts p.N1297K in exon 21 in the interdomain loop III-IV [Fontaine et al., 1990]. The N1297K was absent in more than 100 controls. This mutation was absent in the parents, which suggested a de novo mutation.
DISCUSSION
Here, we report a patient with non-dystrophic myotonia and a severe and early clinical presentation, which made diagnosis difficult. Cold-induced myotonia and attacks of weakness are characteristic of this condition, which explains the significant cooling test result in our patient. However, her neonatal onset, daily episodes of severe desaturation, early-onset muscle hypertrophy, psychomotor retardation, and a fatal outcome are exceptional in non-dystrophic myotonia secondary to SCN4A mutations. Muscle hypertrophy is frequently observed in Thomsen and Becker congenital myotonia, associated with mutations in the skeletal muscle chloride channel gene (CLCN1), but more rarely in myotonia secondary to SCN4A mutations [Colding-Jorgensen, 2005; Vicart et al., 2005]. Psychomotor retardation could be explained by recurrent hypoxemia secondary to myotonic episodes since birth and the very long stay in intensive care. It was suspected that diaphragmatic and laryngeal muscle myotonia was responsible for the attacks of weakness and the episodes of severe desaturation with respiratory blockade.
Because myotonia could not be diagnosed clinically, electromyography was the most informative exam. Serum potassium levels were normal but were not measured during attacks of myotonia or weakness. Muscle biopsy was also non-specific and did not reveal vacuolar changes or abnormalities in proportions of fiber-type as previously described [Heene et al., 1986; Brancati et al., 2003; Miller et al., 2004]. Myotonia and hip dislocation led to the suspicion of Schwartz–Jampel syndrome, which was ruled out by normal perlecan sequencing results on fibroblasts. Because of electromyographic myotonia, Steinert dystrophic myotonia was also ruled out. Sudden attacks of weakness are consistent with hyperekplexia, but GLRA1 gene sequencing analysis was also normal.
Another report of PC-HYPP overlap syndrome with severe early onset was described in an Italian kindred with nine affected individuals [Brancati et al., 2003]. The onset of weakness episodes began in the first year of life and persisted into adult life. Patients showed minimal paramyotonia, mainly of the hands and eyelids, calf hypertrophy, and severe periodic paralysis with several episodes a day lasting for hours. The paralytic episodes were refractory to treatment. All affected family members carried a point mutation of the SCN4A gene, which predicts p.T704M. In contrast to the present report, episodes of severe desaturation with respiratory arrest have never been described in patients with SCN4A mutations.
Some treatments have proven efficacy in myotonic sodium channelopathies, by blocking the sodium channel. These include local anesthetics and class 1b antiarrhythmic agents such as Lidocaine, Mexiletine, and other Lidocaine-derivates [Jackson et al., 1994; Meola and Sansone, 2000]. Carbamazepine and Diphenylhydantoïne can also be used, though with less efficacy [Sechi et al., 1983]. The appropriate dose of Mexiletine for such myotonia in the neonatal period remains unknown. The reported minimal effective dose was 15 mg/kg/ 4 times a day between meals in a 4-month-old child with Thomsen-Becker myotonia [Leheup et al., 1986]. The child was periodically monitored by electrocardiogram. In our observation, Mexiletine was increased progressively because of the undesirable side effects including polymorphic ventricular arrhythmias. Mexiletine therapy and optimal temperature of the room (28°C) were successful in reducing the incidence of myotonia and weakness attacks.
The de novo heterozygous c.3891C > A SCN4A mutation, which predicts N1297K has not been previously described. This missense mutation is predicted to modify a highly conserved amino acid of the interdomain loop III–IV, in which other mutations have been associated with PC or sodium channel myotonias [Vicart et al., 2005]. The intracellular loop between domains III and IV is known to be crucial for fast inactivation by occluding the inner mouth of the pore. To date, PC has been associated with 15 mutations in SCN4A predicting amino acid substitutions at 10 positions in the sodium channel α-subunit [Vicart et al., 2005; Wu et al., 2005; Ferriby et al., 2006]. All were missense mutations resulting in gain-of-function effects.
In conclusion, this unusual case of a patient with severe non-dystrophic myotonia diagnosed in the neonatal period highlights the high clinical variability of sodium channelopathies. It also points to the importance of electromyography in unexplained attacks of weakness in neonates. In addition, although non-dystrophic myotonias are usually non-progressive and do not reduce life expectancy, and although some medications are available that may be effective in alleviating myotonic symptoms, our observation of a fatal outcome shows that the neonatal forms may have a poor prognosis, and that their treatment may be difficult.
Acknowledgements
We thank RESOCANAUX for its contribution to the patient's diagnosis.




