Nonsense-mediated decay and the molecular pathogenesis of mutations in SALL1 and GLI3†
How to cite this article: Furniss D, Critchley P, Giele H, Wilkie AOM. 2007. Nonsense-mediated decay and the molecular pathogenesis of mutations in SALL1 and GLI3. Am J Med Genet Part A 143A:3150–3160.
Abstract
Mutations in SALL1 and GLI3 are responsible for human limb malformation syndromes. The molecular pathophysiology of these mutations is incompletely understood, and many conclusions have been drawn from studies performed in the mouse. We identified truncating mutations in SALL1 and GLI3 in patients with limb malformation and studied the contribution of nonsense-mediated decay (NMD) to the expression of mutant mRNA in patient-derived fibroblasts. Quantification of the relative proportions of mutant and wild-type alleles was performed by pyrosequencing. In SALL1, a mutant allele causing Townes–Brocks syndrome was unexpectedly resistant to NMD, whereas a different mutation causing a much milder phenotype was susceptible to NMD. In GLI3, all three mutant alleles tested were susceptible to NMD. This work provides novel insights into the molecular pathophysiology of SALL1 and GLI3 mutations, extends the phenotypic spectrum of SALL1 mutations, and provides an example of a human mutation which does not follow the usual accepted positional rules governing mammalian NMD. © 2007 Wiley-Liss, Inc.
INTRODUCTION
SALL1 and GLI3 are genes encoding zinc-finger transcription factors that have been implicated in the pathogenesis of dominantly inherited human limb malformations. Heterozygous intragenic truncating mutations in SALL1 cause Townes–Brocks syndrome (TBS), which is characterized by abnormalities of the anus, radial ray, ear, heart, kidney and genitourinary system, and rarely the brain [Kohlhase et al., 1998; Botzenhart et al., 2007]. Three complete SALL1 deletions have also been described; however, the phenotype was mild compared to classical TBS [Borozdin et al., 2006].
Heterozygous mutations in GLI3 have been reported in Greig cephalopolysyndactyly (GCPS), characterized by postaxial polydactyly of the hands, preaxial polydactyly of the feet, syndactyly, macrocephaly, and hypertelorism [Vortkamp et al., 1991; Wild et al., 1997]; Pallister–Hall syndrome (PHS), characterized by central polydactyly of the hands, hypothalamic hamartoma, and other features including postaxial polydactyly of the hands, imperforate anus, and clefting of the larynx [Kang et al., 1997]; and (rarely) acrocallosal syndrome, characterized by postaxial polydactyly of the hands, preaxial polydactyly of the feet, and agenesis of the corpus callosum [Elson et al., 2002]. Patients with GLI3 mutations and limb malformations who lack other syndromic manifestations have been classified with postaxial polydactyly types A and B (PAP-A, PAP-B), characterized by the presence of a well formed extra digit on the ulnar border of the hand, with or without an extra metacarpal (type A), or a rudimentary extra digit (type B) [Radhakrishna et al., 1997]; and preaxial polydactyly type IV, characterized by preaxial polydactyly of the hands and feet, with simple syndactyly of the 3rd web space of the hands and the 2nd web space of the feet [Radhakrishna et al., 1999].
For both genes, genotype–phenotype correlations in mouse and human give clues to the molecular pathogenesis of the underlying mutations. For example, the original Sall1 heterozygous knockout mouse did not phenocopy human TBS [Nishinakamura et al., 2001], but a mouse heterozygous for a mutant allele, that produces a truncated protein, recapitulated the abnormalities found in human TBS [Kiefer et al., 2003]. This suggests that an abnormal truncated SALL1 protein (acting in a dominant negative or gain-of function fashion) is responsible for the full spectrum of developmental defects seen in TBS, whereas haploinsufficiency for SALL1 causes a milder TBS-like phenotype. A more distinct pattern of genotype–phenotype correlation for truncating mutations of GLI3 was reported by Johnston et al. 2005. Those truncations which occurred either within the first 1997 nucleotides of the GLI3 coding sequence, or after nucleotide 3481, caused GCPS, whilst those which occurred between these two nucleotide positions caused PHS, with the notable exception of a recurrent mutation 2374C > T (R792X), which was associated with GCPS. Transfection of mutant cDNA constructs into cell lines suggested that GCPS is caused by functional haploinsufficiency of GLI3, whereas in PHS and PAP-A/B, abnormal truncated GLI3 protein acts in a dominant negative manner [Shin et al., 1999].
In studying the pathogenesis of truncating mutations, an important factor that may contribute to phenotype variability is the extent to which the mutant transcripts may be unstable, thus reducing the production of mutant truncated proteins. Several mechanisms exist within cells to ensure that only correctly processed and error-free mRNA is translated. These methods include a nuclear quality control mechanism which degrades improperly processed mRNA, and nucleo-cytoplasmic mechanisms which target transcripts containing no termination codon (nonstop-mediated decay), or a premature termination codon (PTC; nonsense-mediated decay [NMD]) for degradation. These processes protect the cell from the accumulation of potentially toxic aberrant proteins [Fasken and Corbett, 2005; Khajavi et al., 2006].
The effects of NMD are likely to be significant in the pathogenesis of genetic disease, because approximately one-third of all point mutations that cause genetic disorders are frameshift or nonsense mutations [Mendell and Dietz, 2001]. For example, unusual cases of dominantly inherited β-thalassemia were shown to be related to the lack of mRNA instability when a nonsense mutation lay in the terminal 3′ exon of the HBB gene [Hall and Thein, 1994]. Similarly in SOX10, distinct neurological phenotypes are conveyed by truncating mutations in the 5′ or 3′ portions of the transcript, and the phenotypic variability is mediated by differences in NMD: nonsense mutations in the 5′ part of the gene are susceptible to NMD and cause a milder phenotype (Waardenburg–Shah syndrome) by haploinsufficiency, whereas nonsense mutations in the 3′ part of the gene, which are resistant to NMD, cause a more complex neurocristopathy by a dominant negative mechanism [Inoue et al., 2004]. However, the contribution of NMD to molecular pathogenesis of truncating mutations either in SALL1 or in GLI3 has not, to our knowledge, previously been formally published.
In this report we describe a patient with a frameshift mutation in SALL1 who has an isolated unilateral limb malformation without other organ system involvement, thereby extending the phenotypic spectrum associated with SALL1 mutations. Furthermore, we describe two novel mutations in GLI3 and the results of studies to determine the relative levels of expression of wild-type and PTC-containing mutant alleles of SALL1 and GLI3. These results provide insight into the molecular pathophysiology of SALL1 and GLI3 mutations, and raise further questions with regard to the fundamental mechanism of NMD.
MATERIALS AND METHODS
Ascertainment of Patients
Approval for this work was obtained from the Oxfordshire Research Ethics Committee C (C99.181). Patients were recruited from the pediatric hand surgery clinic at Oxford Radcliffe Hospitals NHS Trust between 1999 and 2006, as part of a larger study into the genetic basis of human limb malformations. All patients or families who presented to the clinic with a congenital limb malformation requiring reconstructive surgery, and who gave informed consent, entered the study. At operation, a maximum of 5 ml of venous blood was collected, from which genomic DNA was isolated using phenol/chloroform extraction. Additionally, if any spare skin was available from the surgery, this was collected in Hank's Balanced Salt Solution (HBSS, Cambrex Bio Science, Verviers, Belgium) and used to set up a fibroblast culture using complete medium.
Mutation Detection
Genomic DNA was amplified by PCR as previously described [Johnson et al., 2003]. All primer sequences and reaction conditions are available on request. Genomic DNA was screened for heterozygous mutations using WAVE denaturing high performance liquid chromatography (dHPLC) (Transgenomic, Elancourt, France), and any abnormally eluting fragments were subjected to DNA sequencing as previously described [Johnson et al., 2003]. Mutations were confirmed using restriction endonuclease digestion. Genbank accession numbers for the reference sequences used were NM_002968 (SALL1) and NM_000168 (GLI3); cDNAs are numbered starting at the A of the initiation codon.
Cycloheximide Treatment of Fibroblasts and RNA Extraction
For quantification of NMD, two 150 cm2 flasks of fibroblast cells grown to confluence were obtained. Medium was poured from the first flask, and replaced by 100 µg of cycloheximide dissolved in 10 ml of a 1% dimethylsulfoxide solution in complete medium (final concentration of cycloheximide 10 ng/µl). The second flask was treated with complete medium only. Both flasks were then incubated at 37°C for 4 hr, after which the medium was poured from both flasks. Cells were rinsed in sterile phosphate buffered saline (Cambrex Biowhitaker) and stored at −70°C in 1 ml of TRIzol (Invitrogen, Paisley, UK). When required, samples were defrosted at room temperature and divided into two aliquots for RNA extraction; 400 µl of chloroform was added to each aliquot and samples were vortexed vigorously prior to centrifugation at 10,400g for 3 min at room temperature. The upper aqueous phase was collected and RNA precipitation performed using the RNeasy kit (QIAGEN, Crawley, UK), following the manufacturer's instructions, from step 4 onwards. Both aliquots of each sample were processed through the same column to allow repooling, and RNA was eluted in a final volume of 30 µl. The RNA concentration was determined by spectrophotometry. Reverse transcription (RT) with random decamers was performed on 1–2 µg of RNA using the RETROscript kit (Ambion/Applied Biosystems, Warrington, UK) according to the manufacturer's instructions. Two microliters of the RT product was used in subsequent PCR amplification.
Quantification of NMD by Pyrosequencing
Pyrosequencing, performed on the PSQ-HS96A system (Biotage, Uppsala, Sweden), was used to measure cDNA expression levels of alleles containing PTCs in SALL1 and GLI3 relative to the wild-type allele, in the presence and absence of prior incubation with cycloheximide. PCR primer sequences, annealing temperatures, sequencing primer sequences, and nucleotide dispensation orders for the mutations studied are shown in Table I. For pyrosequencing performed in the forward direction, the reverse primer was biotinylated, and vice versa. Single stranded DNA was obtained from 10 µl of PCR product by immobilization on streptavidin-coated sepharose beads (Streptavidin Sepharose high performance, GE Healthcare, Chalfont St. Giles, UK) and NaOH denaturation.
| Gene | Mutation | Direction | Amplification primers (5′ → 3′) | PCR | Sequencing primer | ||
|---|---|---|---|---|---|---|---|
| Forward | Reverse | Annealing temperature (°C) | Additive | ||||
| SALL1 | 995delC | Forward | ACAGAGCCTCGCCAGCCAATCTGCCAGCAT | (Biotin)-TTTCAGAGGACGGGGTGGTAACTGCCGCTG | 64 | CAACACCATCATTC | |
| 995delC | Reverse | (Biotin)-Fa | Ra | 64 | AAGAGCCGCTGTTGGATG | ||
| 3414_3415delAT | Forward | CTGTCTTCCTCTGCCACATCCCCAGTTCTGCTCCC | (Biotin)-TAGAAATGTCATGGGGCCATCCACAGAGAGC | 64 | CCCAAGCAGCACTACTGCAA | ||
| 3414_3415delAT | Reverse | (Biotin)-GTCTGGGCCTCTGTCTTCCTCTGCCACATC | Ra | 64 | CGATGAGGAGAAGGTTTTGC | ||
| GLI3 | 1320-21insT | Forward | GCAGCACTGGCGACCCTGCGCACAACAAGAG | (Biotin)-TGTTTGCTTCGGTCTTTGTCCCCTTCCTCCTTGAC | 56.2 | CCAAGATCAAACCCGAT | |
| 1320-21insT | Reverse | (Biotin)-Fa | Ra | 56.2 | GGGCTGGGGAGGTCTTCA | ||
| 2372delC | Forward | CCCGGCAGGGACCAAATGGATGGAGCACGT | (Biotin)-GCAGGTGTTGTTGGACTGTGTGCCATTTCCTATG | 66.8 | DMSOb | ACAAGTGAATGGAATGTTTC | |
| 2372delC | Reverse | (Biotin)-GGACTCAACCATTTCCACTGCAACCACAGC | GGTGTTGTTGGACTGTGTGCCATTTCCTATG | 61.6 | DMSOb | AGAATGGGGTTCAGTCGCG | |
| 2374C > T | Forward | CCCGGCAGGGACCAAATGGATGGAGCACGT | (Biotin)-GCAGGTGTTGTTGGACTGTGTGCCATTTCCTATG | 66.8 | DMSOb | AACAAGTGAATGGAATGTT | |
| 2374C > T | Reverse | (Biotin)-GGACTCAACCATTTCCAGAGCAACCACAGC | GGTGTTGTTGGACTGTGTGCCATTTCCTATG | 61.6 | DMSOb | GGGTAGAATGGGGTTCAG | |
- a Same as Forward (F) or reverse (R) sequence shown in column immediately above.
- b Dimethyl sulfoxide (10% final concentration).
The peak heights were recorded using PSQ96 SQA software and the values exported into an MS Excel data sheet. Each pyrogram had to pass three quality control criteria to be included in the final calculations. These were the absence of unexpected mutant allele specific peaks in the control samples (mutant/wild-type ratio <0.1); no decrease in peak height as the dispensation progressed, which would make comparisons between early and late peaks invalid; and no unexpected peaks from deliberately blank dispensations. For each pair of peaks chosen for quantification, the mutant/wild-type peak ratio was obtained. At least three independent experiments were performed for each mutation.
RESULTS
Identification of Mutations in SALL1 and GLI3
As part of a study of the prevalence of mutations associated with limb malformations requiring surgical correction, we screened patients for mutations in SALL1 (N = 197) and GLI3 (N = 198) using WAVE dHPLC and DNA sequencing. In this report, we have addressed the pathogenesis of those patients who had truncating mutations in either one of these genes, and in whom fibroblasts were available for functional analysis. Two patients with SALL1 mutations and three with GLI3 mutations matched these criteria.
A summary of the clinical features and mutations identified in the probands is shown in Table II. The clinical features of patient OX3335 (TBS) [independently reported by Botzenhart et al., 2007], and patients OX2879 and OX3536 (GCPS), were typical for their respective disorders. Patient OX2948 presented with isolated right hand preaxial polydactyly, classified as Wassel type 7 [Wassel, 1969] (Fig. 1A). There was no family history of congenital malformation. DNA sequencing of SALL1 revealed the mutation 3414_3415delAT, leading to a frameshift, and predicting the addition of 13 abnormal amino acids before termination (Fig. 1B). The deletion causes the addition of only 13 abnormal amino acids because the patient is also homozygous for a previously described single nucleotide [C/T] polymorphism (SNP) at position 3456 (rs11645288) (Fig. 1C). The mutation was confirmed by BsaAI restriction digest of blood and fibroblast derived DNA, which did not show any evidence of mosaicism for the mutation (Fig. 1D).
| Gene | Patient ID | Sexa | Mutation (exon)b | Predicted amino acid change | Phenotype | Bilateral | Family history | Number of limbs affected | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Hands | Feet | Other | ||||||||
| SALL1 | OX3335 | M | 995delC (2) | P332HfsX10 | Preaxial polydactyly | — | Imperforate anus, rectal atresia, hypospadias, overfolded helices | Yes | No | 2 |
| SALL1 | OX2948 | F | 3414_3415delAT (2) | C1139WfsX14 | Preaxial polydactyly, triphalangeal thumb | — | — | No | No | 1 |
| GLI3 | OX2879 | M | 1320_1321insT (9) | E441X | Preaxial polydactyly | Preaxial polydactyly, syndactyly | — | Yes | No | 4 |
| GLI3 | OX2877 | F | 2372delC (14) | P791RfsX3 | Postaxial polydactyly B | — | — | Yes | Yes | 2 |
| GLI3 | OX3536 | M | 2374C > T (14) | R792X | Postaxial polydactyly B | Preaxial polydactyly | Hypertelorism | Yes | Yes | 4 |
- a M, male; F, female.
- b SALL1 comprises 3 exons and GLI3 comprises 15 exons.
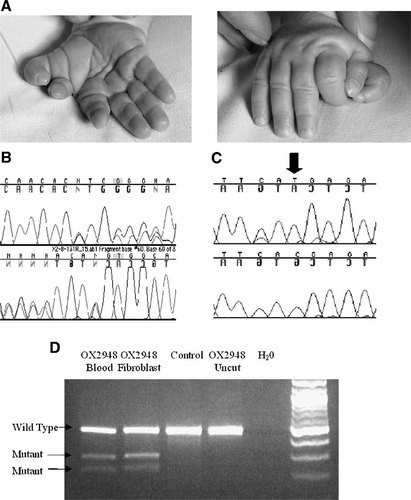
Phenotype and SALL1 gene analysis in patient OX2948. A: Preaxial polydactyly of the right hand, note that the radial thumb is triphalangeal. B: Forward (upper trace) and reverse sequencing of exon 2 reveals a heterozygous dinucleotide deletion, 3414_3415delAT. C: Reverse sequencing of exon 2 from patient OX2948 (upper trace) and control (lower trace) shows a homozygous 3456C > T transition in the patient (arrow). D: BsaAI restriction digest of genomic DNA derived from blood and fibroblasts from patient OX2948 shows no evidence of mosaicism (similar relative intensities of normal and mutant fragments in the two different tissues). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient OX2877 presented with isolated bilateral PAP-B of the hands (Fig. 2A). A family history of PAP-B, stretching back at least three generations was elicited (Fig. 2B). The affected father (II-2) had unilateral PAP-B, and individual II-3, who is predicted to carry the mutation, was reported to be unaffected, demonstrating both incomplete penetrance and variable expressivity of this phenotype. Radiographs of the hands of the proband and her affected father did not reveal any occult central polydactyly. There was no family history of other congenital malformations, in particular there was no history of central nervous system lesions or of unexplained death or miscarriage. DNA sequencing of GLI3 revealed the mutation 2372delC, predicting the addition of two abnormal amino acids before translation prematurely terminates (Table II).
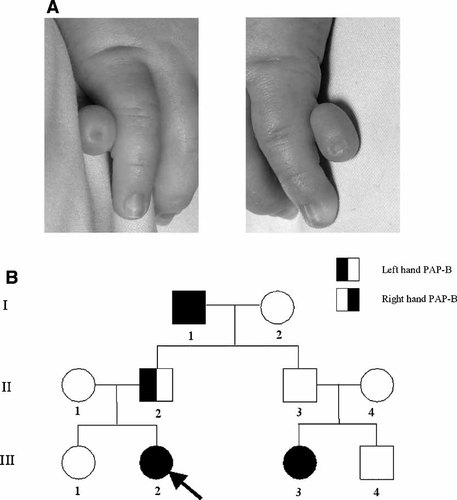
Phenotype and pedigree of patient OX2877. A: The right (left panel) and left (right panel) hands demonstrate bilateral PAP-B. B: Family pedigree; the proband is indicated with an arrow.
Reverse transcriptase-PCR was performed for each mutation, and provided no evidence of abnormal splice variants produced by any of the mutations (data not shown). Furthermore, the creation of neither donor nor acceptor splice sites was predicted by the neural network splice site prediction program (http://www.fruitfly.org/seq_tools/splice) for any of the mutations. Figure 3 shows a schematic of the gene structure of SALL1 and GLI3, together with the position at which each mutation is predicted to truncate the protein, in relation to key functional domains and the position equivalent to the final exon junction.
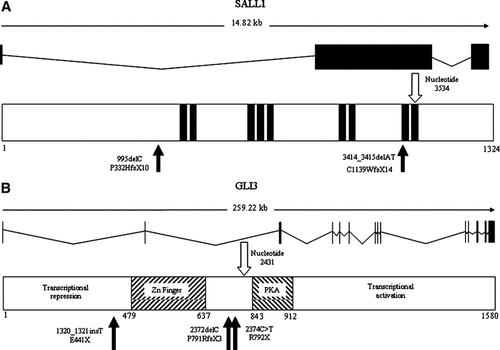
Schematic representation of the gene and protein structure of (A) SALL1 (3 exons) and (B) GLI3 (15 exons; not drawn to scale). In each part, the upper panel shows the genomic and transcript structure and the lower panel shows key functional domains in the protein (filled rectangles in the SALL1 protein represent zinc finger domains). The filled arrows show the positions of mutations described in this report, and the unfilled arrows depict the equivalent positions of the final exon junctions. Zn finger, zinc finger; PKA, multiple phosphokinase A phosphorylation sites.
Quantification of NMD by Pyrosequencing
We used pyrosequencing to provide a highly quantitative measure of the relative amounts of wild-type and mutant alleles in cDNA samples [Langaee and Ronaghi, 2005]. Figure 4 shows typical pyrograms (forward orientation) obtained from SALL1 cDNA prepared from fibroblasts of patients OX3335 (Fig. 4A) and OX2948 (Fig. 4B), respectively, with and without cycloheximide treatment, compared with control fibroblasts. There were no significant mutant-specific peaks in pyrograms from the control samples, in addition these pyrograms passed all other quality control criteria (see Materials and Methods Section). In Figure 4A, the heights of the mutant-specific and wild-type-specific peaks in cDNA from OX3335 are similar, and are unchanged after treatment with cycloheximide. This shows, surprisingly, that the SALL1 mutation 995delC is not subject to NMD. In contrast, inspection of Figure 4B shows that the initial proportion of mutant SALL1 cDNA from patient OX2948 is only ∼40% of the wild-type allele, but treatment with cycloheximide increases this proportion to almost 100%. These data imply that the SALL1 transcript containing the 3414_3415delAT mutation is subject to NMD. We corroborated these findings by pyrosequencing the samples in the reverse orientation; Figure 5 summarizes these data.
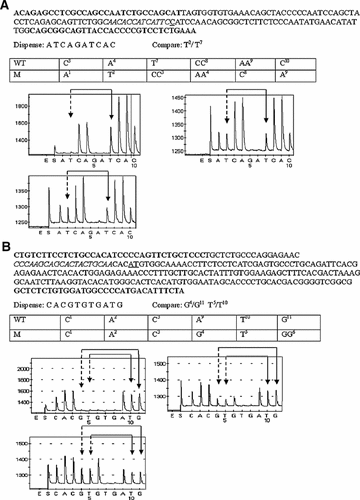
Pyrosequencing analysis of SALL1 cDNA in the forward direction from patients OX3335 (995delC) (A) and OX2948 (3414_3415delAT) (B). In each figure part the cDNA sequence analyzed is shown at the top, with the PCR primer sequences in bold, sequencing primer in italics, and the deleted base(s) underlined. Below, the dispensation order and mutant-(M)/wild-type- (WT) specific bases used for comparison are indicated, with a table listing the allele-specific incorporation of bases at each dispensation (denoted by superscripted numbers). The lower part shows the pyrograms obtained after amplification of untreated cDNA from a control without a SALL1 mutation (upper left), and of untreated (upper right) and cycloheximide-treated (lower left) cDNA from the patient. The mutant allele-specific peaks used for quantification are marked with dashed arrows, wild-type allele-specific peaks are marked with solid arrows.
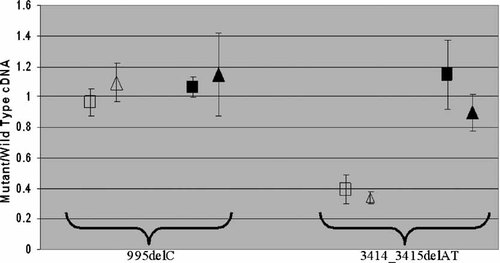
Relative quantification of mutant and wild-type alleles in SALL1 cDNA. The mean proportion (±2 SD) of mutant allele compared to wild-type, before (unfilled symbols) and after (black symbols) cycloheximide treatment, is shown for the 995delC (left) and 3414_3415delAT (right) mutations. Squares and triangles indicate the results of pyrosequencing in the forward and reverse orientations, respectively.
A similar analysis was performed on fibroblasts derived from patients OX2879, OX2877 and OX3536, all of whom have PTC mutations in GLI3 (Fig. 6). By contrast with the variable findings for SALL1, each GLI3 mutation was shown to undergo NMD to a similar extent.
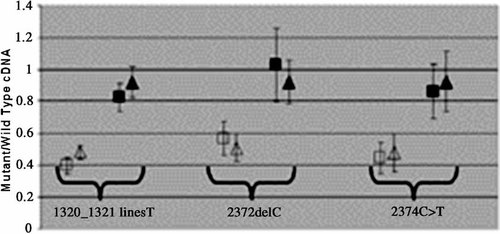
Relative quantification of mutant and wild-type alleles in GLI3 cDNA. The mean proportion (±2 SD) of mutant allele compared to wild-type, before (unfilled symbols) and after (black symbols) cycloheximide treatment, is shown for the 1320_1321insT (left), 2372delC (center) and 2374C > T (right) mutations. Squares and triangles indicate the results of pyrosequencing in the forward and reverse orientations, respectively.
DISCUSSION
In this work, we have analyzed NMD in fibroblasts from two patients with truncating SALL1 mutations and three patients with truncating GLI3 mutations. In four of the five patients (one of two with SALL1 mutations and all three with GLI3 mutations) we demonstrated that the mutant allele was subject to NMD of similar magnitude (cDNA from mutant allele present at 40–50% of wild-type allele, restored to near normal levels after cycloheximide treatment). Unexpectedly, however, in one patient with a SALL1 mutation and typical clinical features of TBS, the mutant allele did not undergo NMD. This identifies an additional mechanism that may influence the phenotype associated with truncating SALL1 mutations.
The two mutations that we studied in SALL1, namely 995delC (P332HfsX8) and 3414_3415delAT (C1139WfsX14), have both been recently described in TBS [Botzenhart et al., 2007]. The 995delC mutation presented with a typical phenotype and is, in fact, the same case as reported by Botzenhart et al. 2007. However, the phenotype of patient OX2948, who presented with an isolated unilateral preaxial polydactyly and has not been reported previously, is milder either than patients with the same mutation reported by Botzenhart et al. 2007, or than the mild phenotype associated with heterozygous SALL1 deletions [Borozdin et al., 2006]. This case extends the phenotypic spectrum of SALL1 mutations, and suggests that the 3414_3415delAT mutation is associated with variable expressivity. The parents of OX2948 were unaffected, but samples were not available for molecular analysis.
Interestingly, the 3414_3415delAT mutation in SALL1 has also been described in a 17-year-old female with bilateral renal hypodysplasia without any extra-renal manifestations [Weber et al., 2006]. The annotation of the mutation described by Weber et al. 2006 (T1138fs1152X) suggests that the stop codon in this patient occurs at the same unusual position as in patient OX2948, and thus that the deletion is inherited in cis with the rare 3456T allele of the rs11645288 SNP. The sequence context of the 3415_3415delAT mutation does not suggest any predisposition to recurrent mutation, making it likely that patient OX2948 and the patient described by Weber et al. 2006 share a common ancestor in whom the deletion occurred in cis with the 3456T allele. It will be important to determine whether the apparently identical mutation described by Botzenhart et al. 2007 occurs in cis to this same unusual allele.
Of the three mutations in GLI3 that we studied, two (1320_1321insT and 2372delC) are newly described, whereas the 2374C > T is a recurrent mutation previously observed in at least six unrelated cases of GCPS [Johnston et al., 2005]. Both the 2372delC and 2374C > T mutations lie in the region of the GLI3 protein in which truncating mutations are typically associated with PHS [Johnston et al., 2005]. Interestingly the 2372delC mutation was associated with a particularly mild phenotype of PAP-B that exhibited both incomplete penetrance and variable expressivity (Fig. 2B). We are only aware of a single previous report documenting incomplete penetrance in a family with a GLI3 mutation [Debeer et al., 2003]. None of the additional features of GCPS or PHS were evident in any of the affected family members.
Our studies of NMD shed light on the pathogenesis, and genotype–phenotype correlations, of both SALL1 and GLI3 truncating mutations. In the case of SALL1, we obtained strikingly different results for the two mutations. The 3414_3415delAT mutation was present at only ∼40% of the level of the wild-type allele, but this was fully restored after cycloheximide treatment (Fig. 5, right). These findings are typical of NMD, and the observed under-representation of the mutant allele is in the range reported in previous studies of naturally occurring PTC mutations [Perrin-Vidoz et al., 2002; Harries et al., 2005]. NMD of the mutant transcript may mitigate the adverse effects of the mutation, consistent with the associated mild and variable phenotype.
By contrast, the level of mutant SALL1 cDNA from fibroblasts derived from patient OX3335 (995delC) with typical TBS was equal to wild-type, and did not change when the cells were treated with cycloheximide. Although it had previously been speculated that truncating TBS mutations might escape NMD [Borozdin et al., 2006], this finding was surprising because the lack of NMD in this case contravenes the rule that it is predicted to occur when the PTC is ≥50–55 nucleotides 5′ of the final exon junction [Zhang and Maquat, 1997]. The 995delC mutation resides in the very large exon 2 of the three exon SALL1 gene, well upstream of this boundary (and indeed upstream of the 3414_3415delAT mutation, which does undergo NMD; Fig. 3). This provides the first direct evidence in humans that TBS may be caused by a truncated SALL1 protein acting in a dominant negative manner. It is consistent with mouse models of TBS [Kiefer et al., 2003], and also validates the in vitro experimental approach using mutant cDNA constructs taken by Sakaki-Yumoto et al. 2006.
Turning to the analysis of GLI3, we obtained similar results with all three PTC mutants, which exhibited mutant cDNA levels of 40–60% of the wild-type allele, that were restored to normal after cycloheximide treatment. Thus all three alleles appear subject to NMD, which is likely to contribute to the milder phenotypes (GCPS and PAP-B) observed in association with these mutations, and may account for why the two truncations in the “PHS region” that we studied (2372delC and 2374C > T) are not associated with any definite PHS features. Our finding of NMD in the case of the 2374C > T mutation differs from unpublished observations on lymphoblast cells from a different patient, in which the level of mutant message appeared similar to wild-type [Johnston et al., 2005], but concords with the GCPS phenotype consistently associated with this recurrent mutation. The demonstration that GLI3 mutations undergo NMD is important, as the in vitro work reported by Shin et al. 1999 assumed that the mutations causing GCPS, PHS, and PAP all lead to the production of truncated proteins, without taking the possibility of NMD into account. Ideally, the contribution of NMD should be assessed in parallel with such functional analyses. We speculate that PHS mutations may be characterized by partial or complete escape from NMD, in a manner analogous to the situation that we have documented for the 995delC mutation in SALL1. Unfortunately no fibroblasts from PHS patients were available to us to test this hypothesis.
Why certain mutations more than 55 nucleotides 5′ of the final exon junction may escape NMD is not well understood, although other examples have been reported [Zhang and Maquat, 1997; Inoue et al., 2004; Harries et al., 2005]. Most of these mutations are located close to the initiation codon, and it is postulated that NMD avoidance is mediated by reinitiation of translation [Zhang and Maquat, 1997; Harries et al., 2005], or by physical proximity to the initiation codon [Inacio et al., 2004]. The 995delC mutation, in common with other mutations reported to cause TBS, is not close to the initiation codon. Indeed, a PTC too close to the initiation codon would yield a truncated protein of insufficient length to possess the dominant negative functions required to give rise to the phenotype. This suggests that a novel mechanism of NMD avoidance is operating in TBS, and further study may provide insights into the basic mechanism of NMD.
Finally, it is important to note that whilst our results are consistent with a role for NMD in the molecular pathology of mutations in SALL1 and GLI3, strictly speaking the experimental method that we used only provides evidence that a process dependent on protein synthesis is involved. The protein synthesis inhibitor used in these experiments, cycloheximide, is indeed widely employed to prevent NMD by preventing the “pioneer round” of translation which detects a PTC [Fasken and Corbett, 2005]. However, strategies to directly inhibit the NMD pathway, for example by RNAi knock down of UPF1, would be required to establish more rigorously the role of NMD in these patients.
Acknowledgements
We are grateful to the families for their cooperation with this study, to D. Johnson for assistance with patient recruitment, S. Butler for fibroblast culture, and K. Clarke for DNA sequencing. Pyrosequencing was conducted using the London IDEAS Knowledge Park facility (Institute of Child Health, London) with the help of K. Pearce, P. Scambler, A. Goriely and S. Twigg. This work was supported by grants from the Wellcome Trust to D.F. (Clinical Training Fellowship) and A.O.M.W. (Programme Grant).




