Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15†
How to cite this article: Boyles AL, Enterline DS, Hammock PH, Siegel DG, Slifer SH, Mehltretter L, Gilbert JR, Hu-Lince D, Stephan D, Batzdorf U, Benzel E, Ellenbogen R, Green BA, Kula R, Menezes A, Mueller D, Oro' JJ, Iskandar BJ, George TM, Milhorat TH, Speer MC. 2006. Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet Part A 140A:2776–2785.
Abstract
Chiari type I malformation (CMI; OMIM 118420) is narrowly defined when the tonsils of the cerebellum extend below the foramen magnum, leading to a variety of neurological symptoms. It is widely thought that a small posterior fossa (PF) volume, relative to the total cranial volume leads to a cramped cerebellum and herniation of the tonsils into the top of the spinal column. In a collection of magnetic resonance imagings (MRIs) from affected individuals and their family members, we measured correlations between ten cranial morphologies and estimated their heritability in these families. Correlations between bones delineating the PF and significant heritability of PF volume (0.955, P = 0.003) support the cramped PF theory and a genetic basis for this condition. In a collection of 23 families with 71 affected individuals, we performed a genome wide linkage screen of over 10,000 SNPs across the genome to identify regions of linkage to CMI. Two-point LOD scores on chromosome 15 reached 3.3 and multipoint scores in this region identified a 13 cM region with LOD scores over 1 (15q21.1-22.3). This region contains a biologically plausible gene for CMI, fibrillin-1, which is a major gene in Marfan syndrome and has been linked to Shprintzen–Goldberg syndrome, of which CMI is a distinguishing characteristic. Multipoint LOD scores on chromosome 9 maximized at 3.05, identifying a 40 cM region with LOD scores over 1 (9q21.33-33.1) and a tighter region with multipoint LOD scores over 2 that was only 8.5 cM. This linkage evidence supports a genetic role in Chiari malformation and justifies further exploration with fine mapping and investigation of candidate genes in these regions. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Chiari type I malformation (CMI; OMIM 118420) is part of a pathological continuum of hindbrain malformations, clinically defined as an inferior displacement of the cerebellar tonsils below the foramen magnum, thought to be caused by a small posterior fossa (PF) volume [Milhorat et al., 1999]. CMI is the most common form of Chiari and the phenotype is continually being refined as more research is conducted on the disorder [Milhorat et al., 1999]. Prior to magnetic resonance imaging (MRI), diagnosis was difficult, so the true incidence of the disorder is only recently being understood [Pillay et al., 1991; Elster and Chen, 1992]. The prevalence may be as high as 1/1280 [Meadows et al., 2000], with the most recent study estimating that 215,000 Americans may be affected [Speer et al., 2003].
Chiari patients usually present with a variety of neurological conditions. A prospective study of 265 patients with CMI reported headaches (98%), dizziness (84%), disequilibrium (46%), neck pain (67%), sleep difficulty (72%), weakness in the upper (69%) or lower (52%) extremities, numbness or tingling in the upper (62%) or lower (43%) extremities, general body weakness (46%), difficulty swallowing (54%), shortness of breath (57%), nausea (58%), blurred vision (57%), depression (47%), tinnitus (56%), memory problems (45%), slurred speech (28%), raspy or hoarse voice (41%), cardiac abnormalities (39%), and facial numbness (32%) [Mueller and Oro', 2004]. These constellations of reported symptoms are not individually unique to Chiari and make diagnosis difficult, particularly when 95% of patients report more than five symptoms [Mueller and Oro', 2004].
One of the more severe conditions concurrent with CMI is syringomyelia observed in 65–80% of patients [Pillay et al., 1991; Milhorat et al., 1999; Ellenbogen et al., 2000]. This condition is an abnormal collection of cerebrospinal fluid (CSF) in a fluid-filled sac within the spinal cord or brain stem [Ellenbogen et al., 2000]. CMI is the leading cause of syringomyelia, a debilitating disorder that can lead to neurological impairments including loss of motor function [Banerji and Millar, 1974; Dyste et al., 1989; Pillay et al., 1991; Milhorat et al., 1993; Milhorat et al., 1995]. Alone, syringomyelia is not significantly associated with occipital-suboccipital pain, the most common type of CMI headache [Pascual et al., 1992], so both disorders contribute to patients' diverse symptoms.
The medical and scientific fields need to establish criteria to distinguish the true malformation of tonsillar ectopia from simply a variant of normal anatomy [Ball and Crone, 1995; Batzdorf, 2001]. Historically, herniation of one tonsil 5 mm below the foramen magnum or herniation of both tonsils 3 mm or more is typically considered affected [Aboulezz et al., 1985; Elster and Chen, 1992]. Herniation less than 2 mm is unlikely to be symptomatic [Barkovich et al., 1986]. An extensive study of 200 healthy adults and children concluded that 3 mm was an appropriate cutoff (96% sensitivity, 99.5% specificity), while 2 mm was less specific (100% sensitivity, 98.5 specificity) [Barkovich et al., 1986]. The extreme heterogeneity of CMI clinical presentation and etiology justifies further examination of the phenotype, considering several diagnostic criteria.
Many morphometric studies support the observation that the PF is small and shallow in CMI patients, compared to the normal population while overall cranial volume is not reduced [Nyland and Krogness, 1978; Schady et al., 1987; Nishikawa et al., 1997; Milhorat et al., 1999]. Discriminant analysis found that PF area and clivus length were able to identify 76% of CMI patients and 79% of controls [Vega et al., 1990]. A study of 50 patients to 50 controls found reduced height of supraocciput (P < 0.001), reduced length of clivus (P < 0.001), increased slope of the tentorium (P < 0.001), tonsillar herniation (9.8 ± 5.8 mm, P < 0.001), reduced mean volume of posterior cranial fossa (P = 0.001), and reduced CSF volume (P < 0.001), yet there was no significant difference in the overall brain volume (P = 0.369) [Milhorat et al., 1999].
Disrupted CSF flow could cause many CMI symptoms and the development of syringomyelia. It is not clear how a cyst is formed, but the normal pulse in flow can dissect the cord and enlarge an existing cyst [Williams et al., 1981]. Treatment that enlarges the foramen magnum and subarachnoid space would disable the piston-like motion of the tonsils. The clinical symptoms of CMI are thought to result from the build-up of scar tissue from years of tonsils rubbing on the foramen magnum, increasing compression, and restricting CSF flow [Barkovich et al., 1986; Milhorat et al., 1999]. Even in patients who do not have syringomyelia, 66% have associated spinal cord disturbances [Milhorat et al., 1999].
Most of the published CMI literature with respect to genetics consists of case reports of familial aggregation [Coria et al., 1983; Herman et al., 1990; Stovner et al., 1992; Caldemeyer et al., 1995; Cavender and Schmidt, 1995; Gripp and Scott, 1995; Wolpert et al., 1996, 1998]. Estimates of incidence vary from 1/18,000 to 1/1280 with accuracy impaired by MRI availability and under-diagnosis of asymptomatic patients [Small and Sheridan, 1996; Meadows et al., 2000; Speer et al., 2003]. There have been eight reported cases of twins with CMI and one set of monozygotic (MZ) triplets [Stovner, 1992; Cavender and Schmidt, 1995; Turgut, 2001; Speer et al., 2003], with a higher concordance observed in MZ twins than dizygotic twins [Speer et al., 2003]. In a study of 364 patients, 12% had at least one close relative with CMI and/or syringomyelia [Milhorat et al., 1999]. A report of MZ twins and additional family members with CMI found that their type of headache segregated in the family as well [Stovner, 1992]. It is unclear, how well other symptoms of Chiari correlate within families.
In 21 pedigrees with two or more members with CMI, several patterns were observed: affected females outnumbered affected males 3:1, transmission from parent to child including male-to-male transmission, transmission through apparently unaffected family members, and recurrence in sibships. These patterns are consistent with autosomal dominant or autosomal recessive inheritance with reduced penetrance [Milhorat et al., 1999]. An unclear genetic model justifies the use of multiple analytical approaches.
Another source of evidence for a genetic component to CMI comes from its association with a variety of other disorders. Ninety-eight cases of CMI have been reported with another genetic condition as of 1998, but this accounts for less than 1% of total CMI cases [Speer et al., 2003]. Many of these known genetic disorders that cosegregate with CMI affect mesodermally derived cartilage and/or bone including achondroplasia, Klippel Feil sequence, Hadju–Cheney syndrome, Albright Hereditary Osteodystrophy (pseudohypoparathyroidism), hypophosphatemia rickets, and spondylo-epiphyseal dyplasia tarda [Pauli et al., 1995; Gripp et al., 1997]. Other known genetic disorders that have been reported to cosegregate with CMI include Crouzon syndrome, cystic fibrosis, neurofibromatosis type I, Paget disease of the bone, and Williams syndrome [Steinberg and Brown, 1960; Grimm and Wesselhoeft, 1980; Parkinson and Hay, 1986; Afifi et al., 1988; Cohen and Kreiborg, 1992; Dooley et al., 1993; Lazaro et al., 1994; Pober and Filiano, 1995; Iglesias-Osma et al., 1997; Mercuri et al., 1997; Needleman et al., 2000; Fujisawa et al., 2002; Caldarelli and Di Rocco, 2004; Tubbs et al., 2004].
Characterizing the phenotypic presentation and genetic contribution to CMI could yield very valuable insight into the complex etiology and pathogenesis of the disorder. Treatment options could be more appropriately matched to patients if there were a better understanding of who would benefit most from surgical intervention. Such work would provide genetic counselors with a clearer understanding of the risk of symptoms and risk of CMI in relatives of affected patients. Such work has the opportunity to elucidate the cause of CMI and directly benefits patient outcomes.
MATERIALS AND METHODS
Patient Ascertainment
Families were ascertained for this study from a variety of locations throughout the United States. MRIs were acquired and included in the study of cranial morphology if available films were taken prior to decompression surgery and the subject was at least 2 years of age at the time of the MRI. Blood samples were collected by trained phlebotomy staff from affected individuals and their first degree relatives, plus any additional individuals necessary to connect affected individuals within one family. In the genomic screen dataset, 23 Caucasian American families were used including 67 sampled individuals with CMI. Only 12 of the 35 families with utilized MRIs were included in linkage analysis because multiple affected individuals were not sampled or successfully genotyped in the other families. Figure 1 displays the genomic screen families. For the linkage analysis, affection status is based on patient report of CMI, the phenotype with the largest dataset. One subject is indicated with a ? due to a diagnosis of Chiari type 0, indicating that he has many of the classic symptoms of Chiari without herniation of the tonsils over 5 mm. More restrictive diagnostic criteria including the traditional diagnoses of ≥3 mm herniation by our review of MRIs, ≥5 mm herniation by our review of MRIs, and confirmation by medical records and/or MRI review were also used in linkage analysis, but the sample sizes were too small to yield robust results (data not shown). A confirmed herniation over 3 mm only included 13 families, and only 12 families had more than two members with a herniation over 5 mm. Blood samples, medical records, and MRIs were obtained through informed consent under the approval of the Duke University Medical Center Institutional Review Board.
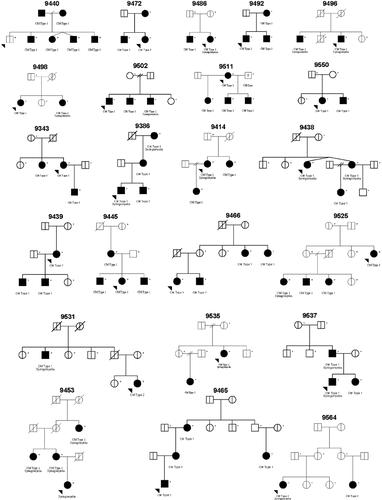
Pedigree structure of families used in the genomic screen dataset. Shaded = affected with Chiari type I malformation defined by patient report; unshaded = confirmed normal by MRI; ? = Chiari 0 diagnosis; vertical line = no confirmed information on affection status; + = sampled and genotyped individuals.
Phenotypic Measurements From MRIs
To investigate inter-rater reliability, ten morphologies were independently measured using the ImageJ 1.31v software package [Rasband, 2004] in a series of 14 randomly selected MRIs by two trained researchers. While there was a strong correlation between the observers (90% for the degree of herniation, 95% on volume measurements), if there was a discrepancy greater than 10% for linear measurements, 4% for angles, and 2% for volumes, individual measurements were independently remeasured once. An initial training set of 14 MRIs was comparable to those also evaluated by a board certified neuroradiologist (DSE). In the final data set, the average correlation between the two observers was 0.91 for herniations, 0.79 for the other sagittal view measurements, and 0.96 for volumes.
Herniation of the left and right tonsils was measured on a line drawn from the tips of the cerebellar tonsils perpendicularly to a line between the basion and the opisthion on a sagittal image to the left and right of midline, respectively. All measurements taken in the sagittal view are illustrated in Figure 2. The following linear and angular measurements were measured on a midline sagittal image. The foramen magnum was measured as the distance from the basion to the opisthion. The length of the tentorium was measured from its anterior midline, just posterior to the vein of Galen to the internal occipital protuberance. The lower portion of the occipital bone, the supraocciput, was measured from the center of the internal occipital protuberance to the opisthion. The slope of the tentorium was measured as the angle formed by the tentorium and supraocciput. The clivus, including the basiocciput and basisphenoid, was measured from the top of the doral sella to the basion. Basal angulation was evaluated as the angle formed by a line from the basion to the center of the sella turcica and a line from the center of the sella turcica to the nasion.
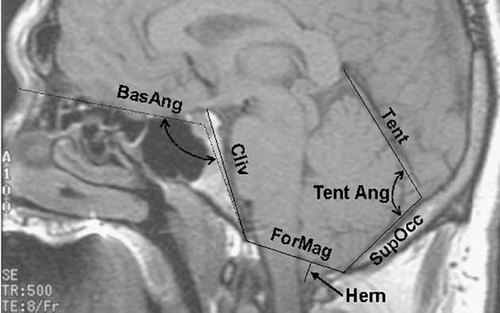
Cranial morphologies measured on a sagittal MRI image. Hern = herniation; ForMag = foramen magnum; Tent = tentorium; SupOcc = supraocciput; TentAng = tentorium angle; Cliv = clivus; BasAng = basal angulation.
Cranial volume calculations were made from serial axial images, taken from the foramen magnum to the top of the skull. The area of the PF and total cranium were measured on each image. To calculate the volume, each area was multiplied by the slice thickness. The area between two sequential slices was calculated as the mean value of the neighboring slice areas multiplied by the skipped distance between slices, and all of these values were summed for total cranial and PF volume.
The entire dataset was used to estimate correlations between structural measurements, this included, 113 individuals (70 female, 43 male) with an average age at MRI of 31.1 years (range: 2.6–73.6). Correlations were calculated with SAS 8.1 (SAS Institute, Inc., Cary, NC). Only MRIs from multiple members of a family were included in estimates of polygenic heritability, including a one-tailed chi square test comparing the polygenic model to a sporadic model using SOLAR 2.1.2 [Almasy and Blangero, 1998]. This included 99 subjects from 35 families (61 female, 38 male) with an average age of 30.3 years and the same age range. The available MRI views varied between individuals, so not all ten morphologies were able to be assessed on all subjects. In the total dataset of 113 subjects, 108 were evaluated for herniation (95 of 99 in the family set), 104 had measurable tentoriums (93 in families), in 105 subjects all other sagittal view measurements were taken (93 in families), and complete volumes were calculated on 85 subjects (74 in families).
Genotyping Methods
For inclusion in the genomic screen, a family needed to have at least two sampled and genotyped individuals affected with CMI by patient report. DNA was extracted from the whole blood samples with the Puregene system (Gentra Systems, Minneapolis, MN). Translational Genomics performed the genotyping using the whole-genome Affymetrix 10K SNP Chip (TGen, Phoenix, AZ). This system yielded genotypes for approximately 10,644 SNPs at an average density of 1 SNP every 210 kb. Mendelian pedigree inconsistencies were identified with PEDCHECK [O'Connell and Weeks, 1998] and familial relationships confirmed with RELPAIR [Boehnke and Cox, 1997; Epstein et al., 2000].
Linkage Analysis
Since the underlying genetic model for CMI is unknown, we used both a parametric model (assuming dominant inheritance, 0.0005 prevalence, and 80% penetrance) and nonparametric models to assess linkage. The pedigrees in our study were consistent with a high penetrance, such as 0.8, and in this type of almost “affecteds only” type analysis, the penetrance would have to be substantially misspecified to significantly impact linkage analysis [Clerget-Darpoux et al., 1986]. The ALLEGRO v1.2 software package can perform both of these analyses including both two-point (disease and one marker) and multipoint (disease and many markers) tests [Gudbjartsson et al., 2000]. Markers in our dataset with a genotyping efficiency less than 85% or heterozygosity less than 0.05 were removed for potential lack of informativeness. With high levels of inter-marker linkage disequilibrium (LD) expected between some SNPs, given the density of this screen, we reduced the number of markers in LD to avoid inflating the false positive rate in multipoint linkage analysis [Boyles et al., 2005] by selecting only one SNP from groups of markers with elevated LD (r2 > 0.16), using ldselect v1.0 [Carlson et al., 2004]. Since the families in our dataset were of various sizes and structures, it is most aptly suited to the Spairs statistic to assess identity by descent sharing between all pairs of affected individuals [Whittemore and Halpern, 1994; Gudbjartsson et al., 2000], and we included both the linear and exponential models as implemented in ALLEGRO [Kong and Cox, 1997].
RESULTS
Cranial Morphologies Are Moderately Correlated
The full set of MRI data was used to calculate correlations between cranial morphologies while heritability estimates used the family subset. Table I includes the comparable means, standard deviations, and ranges of values for both datasets. The highest correlations were found between the herniation measurements (left-right = 0.921, left-maximum = 0.954, and right-maximum = 0.985), as seen in Table II. PF volume was also strongly correlated with total cranial volume (0.729) and the calculated PF/supratentorial volume ratio (0.623). A high positive correlation was also observed between PF volume and clivus length (0.427), while a negative correlation exists between the angle of the tentorium and supraocciput length (−0.469). The major structures surrounding the PF were correlated with PF and total cranial volume (foramen magnum-PF = 0.331, tentorium-PF = 0.277, tentorium-total volume = 0.290, supraocciput-PF = 0.283, and supraocciput-total volume = 0.258).
| Mean | St. Dev | Range | |
|---|---|---|---|
| Full data | |||
| Left herniation (mm) | 5.79 | 4.08 | 0–15.7 |
| Right herniation (mm) | 6.00 | 4.52 | 0–18.3 |
| Foramen magnum (mm) | 36.2 | 3.41 | 26.4–46.7 |
| Tentorium (mm) | 51.8 | 4.27 | 42.2–63.5 |
| Supraocciput (mm) | 39.2 | 4.58 | 25.4–52.1 |
| Tentorium angle (°) | 91.6 | 8.11 | 74.1–112.2 |
| Clivus (mm) | 41.5 | 4.13 | 28.8–51.7 |
| Basal angulation (°) | 127.5 | 5.33 | 116.1–142.4 |
| Posterior fossa volume (cc) | 196.0 | 22.1 | 147.2–285.4 |
| Cranial volume (cc) | 1426 | 127.3 | 1169–1663 |
| Family subset | |||
| Left herniation (mm) | 6.41 | 3.60 | 0.72–15.7 |
| Right herniation (mm) | 6.69 | 3.78 | 0.55–16.2 |
| Foramen magnum (mm) | 36.6 | 2.85 | 28.2–44.7 |
| Tentorium (mm) | 51.7 | 4.50 | 42.2–63.5 |
| Supraocciput (mm) | 38.7 | 3.96 | 30.4–51.1 |
| Tentorium angle (°) | 92.0 | 7.81 | 74.1–108.3 |
| Clivus (mm) | 41.4 | 4.29 | 32.7–51.7 |
| Basal angulation (°) | 127.8 | 4.96 | 117.7–140.6 |
| Posterior fossa volume (cc) | 195.3 | 18.6 | 147.2–238.9 |
| Cranial volume (cc) | 1425 | 126.4 | 1169–1641 |
| LH | RH | MH | FM | TE | SO | TA | CL | BA | PF | CR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RH | 0.921 | ||||||||||
| MH | 0.954 | 0.985 | |||||||||
| FM | −0.068 | −0.019 | −0.045 | ||||||||
| TE | 0.173 | 0.123 | 0.146 | 0.105 | |||||||
| SO | −0.115 | −0.163 | −0.160 | −0.258 | 0.038 | ||||||
| TA | 0.024 | 0.068 | 0.040 | 0.223 | −0.057 | −0.469 | |||||
| CL | −0.238 | −0.195 | −0.223 | 0.087 | 0.173 | 0.045 | 0.284 | ||||
| BA | 0.197 | 0.189 | 0.200 | −0.041 | −0.013 | 0.017 | 0.027 | −0.134 | |||
| PF | −0.124 | −0.047 | −0.090 | 0.331 | 0.277 | 0.283 | 0.077 | 0.427 | −0.172 | ||
| CR | 0.007 | −0.012 | −0.022 | 0.247 | 0.290 | 0.258 | −0.054 | 0.316 | −0.157 | 0.729 | |
| PS | −0.180 | −0.047 | −0.098 | 0.203 | 0.064 | 0.102 | 0.171 | 0.263 | −0.078 | 0.623 | −0.078 |
- RH = right herniation; LH = left herniation; MH = maximum herniation; FM = foramen magnum; TE = tentorium; SO = supraocciput; TA = tentorium angle; CL = clivus; BA = basal angulation; PF = posterior fossa volume; CR = cranial volume; PS = posterior fossa/supratentorial volume ratio.
Posterior Fossa Volume Is Highly Heritable
By using the subset of MRI measurements from multiple family members, we determined the heritabilities of the morphologies in our population, as displayed in Table III. PF volume was highly heritable (0.955, P = 0.0035), while herniation was not heritable at all. Heritability of basal angulation was also significant (0.513, P = 0.0143), while clivus (0.388, P = 0.0542) and supraocciput (0.277, P = 0.0685) were approaching significance. The reported P-values are from a one-tailed chi square test, comparing the polygenic model to a sporadic model. Kurtosis levels were within acceptable limits for all measurements except foramen magnum (1.8) and all values were within 3.2 standard deviations of the mean, indicating that the other measurements are approximately normally distributed and the polygenic model used in SOLAR was appropriately applied.
| H2r | P-value | |
|---|---|---|
| Left herniation (mm) | 0 | 0.5 |
| Right herniation (mm) | 0 | 0.5 |
| Maximum herniation (mm) | 0 | 0.5 |
| Foramen magnum (mm) | 0.188255 | 0.2744 |
| Tentorium (mm) | 0.110064 | 0.3089 |
| Supraocciput (mm) | 0.277443 | 0.0685 |
| Tentorium angle (°) | 0.101621 | 0.3875 |
| Clivus (mm) | 0.387862 | 0.0542 |
| Basal angulation (°) | 0.512604 | 0.0144 |
| Posterior fossa volume (cc) | 0.955257 | 0.0035 |
| Cranial volume (cc) | 0.105482 | 0.3240 |
- P-values from a one-tailed chi square test comparing the polygenic model to a sporadic model are reported, with significant P-values (<0.05) in bold.
Significant Two-Point Linkage to 15q21.1-22.3
At rs744318, the highest two-point LOD score of 3.332 was attained on the q arm of chromosome 15. Figure 3 displays the two-point parametric LOD scores over 0.5 accounting for heterogeneity (HLOD) and nonparametric LOD scores for all chromosomes. The exponential model produced LOD scores over 2 at three SNPs on chromosome 15q21.3: rs744318 (3.33), rs1908205 (2.73), and rs956215 (2.05). The parametric model also produced HLOD scores over 2 at this location: rs744318 (2.03), rs1908205 (2.29), and rs956215 (0.79). LOD scores under the linear model were not over 2, but they were higher for these SNPs than on any other chromosome: rs744318 (1.83), rs1908205 (1.59), and rs956215 (1.43).
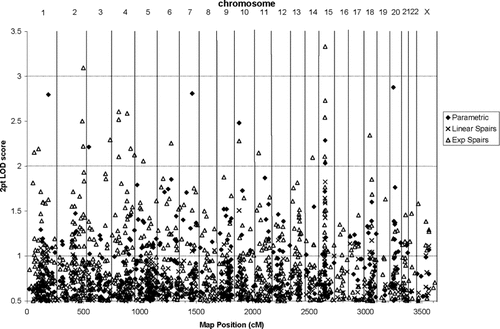
Two-point linkage results over 0.5. Solid diamond = Parametric dominant model HLOD score; X = Linear model, Spairs statistic LOD; open triangle = Exponential model, Spairs statistic LOD; the y-axis indicates the LOD or HLOD score while the x-axis indicates the genetic distance in cM using a Kosambi map. Chromosome number is indicated along the top of the graph.
Multiple markers were incorporated into the LOD score through multipoint linkage analysis and produced a peak approaching 2.5, just upstream of the high two-point results on chromosome 15. Figure 4 graphs multipoint and two-point results for the four regions with multipoint scores over 1.5. All three analyses on chromosome 15 peaked between SNPs rs959638 and rs400215: exponential (2.458), linear (1.808), and parametric (0.7756). The region on chromosome 15 where the exponential model Spairs LOD score was over 1 spans a 13 cM area from rs1439331 to rs931183. The average information content in this region is 0.955 (ranging from 0.866 to 0.992).
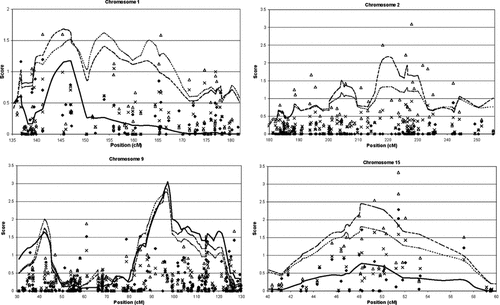
Two-point and multipoint linkage results in regions with a multipoint score greater than 1.5. Solid diamond, solid line = two-point and multipoint under a parametric dominant model HLOD score; X, dotted line = two-point and multipoint under a linear model, Spairs LOD; open triangle, dashed line = two-point and multipoint under an exponential model, Spairs LOD; the y-axis indicates the LOD or HLOD score while the x-axis indicates the genetic position on the chromosome in cM using a Kosambi map.
Significant Multipoint Linkage to 9q21.33-33.1
The highest multipoint linkage score was attained at 9q22.31, despite having no two-point HLOD scores over 2 in this region. The multipoint scores peaked between rs1000735 and rs2895201: parametric model (3.05), linear model (2.86), and exponential model (2.77). Two-point scores in this region did not exceed 1. The region around this peak where the LOD score is over 1 spans 40 cM from rs534517 to rs2418400. A narrower region above a LOD score of 2 is only 8.5 cM from rs1573231 to rs1412488. The 1 LOD region averaged 0.940 information content (0.859–0.978), while the narrower 2 LOD region was lower (0.900 ranging from 0.864 to 0.958).
Other Regions of Marginally Significant Linkage
At 9p21.3, multipoint linkage scores reached 2 near rs1111766: linear model (2.004), exponential model (1.755), and parametric model (1.6431). A large 1 LOD region including the parametric multipoint spans 13 cM, but a narrower peak defined by the nonparametric methods is only 7 cM from rs947144 to rs1410845. Figure 4 also included regions on 1q and 2q where multipoint LOD scores exceeded 1.5, but neither of these regions has as strong evidence for linkage as chromosomes 15 and 9. Other regions, where parametric two-point scores approached 3 (1q31.2 rs1408830 = 2.80, 7q31.1 rs958840 = 2.81, and 20p12.2 rs973542 = 2.88), did not have nearby scores approaching that level and multipoint LOD scores were not significant.
DISCUSSION
Chiari type I malformation is thought to be the result of a cramped PF, leading to herniation of the cerebellar tonsils through the foramen magnum [Nyland and Krogness, 1978; Schady et al., 1987; Nishikawa et al., 1997; Milhorat et al., 1999]. Unlike other forms of Chiari malformation, CMI is not diagnosed at birth and is associated with a heterogeneous mixture of symptoms. Correlations between PF volume and the major bones that delineate it, support the cramped PF theory [Batzdorf, 2001]. The clivus, foramen magnum, supraocciput, and tentorium had correlations with PF volume over 0.25. The angle of the tentorium was not correlated with PF volume, but it did have a high positive correlation with the length of the clivus and a negative correlation with the length of the supraocciput—both of which contribute to the PF volume.
The very high heritability calculated for PF volume, combined with no evidence for heritability in herniation, may indicate that the genetic contribution to CMI in our families is derived from the size of the PF, with herniation secondary to hindbrain cramping. Other morphologies contributing to PF shape, such as basal angulation, clivus length, and supraocciput length, were moderately heritable and could contribute to a genetic basis as well. A larger dataset may yield more precise estimates of these values, as underlying etiological heterogeneity may lead to an underestimation. In our dataset, there was not enough overlap between available MRIs and available blood samples to warrant attempts to look for genetic determinates of these quantitative traits.
DNA samples were available on a large enough set of families to identify two significant regions of linkage on chromosomes 9 and 15. Two-point LOD scores on chromosome 15 were over 3 and multipoint LOD scores in the region identified a 13 cM region with a LOD score over 1. This region spans 12.3 Mb containing 71 genes and 3 predicted genes (Ensembl v37). A smaller region with a LOD score over 2 is only 4 cM, 4.7 Mb, and contains 16 genes and 1 predicted gene. The linked region over 1 on chromosome 9 is much larger: 40 cM, 31.3 Mb, 193 genes, and 3 predicted genes. The much tighter linkage peak over 2 may be more manageable to follow-up since it is only 8.5 cM, 6.18 Mb, and contains 41 genes plus 1 predicted gene.
One of the genes in the linked region on chromosome 15 is a plausible candidate gene for CMI due to its known role in related genetic disorders. Fibrillin-1 (FBN1) is the major cause of Marfan syndrome [Dietz et al., 1991] and has also been linked to ectopia lentis and Shprintzen–Goldberg syndrome [Tsipouras et al., 1992; Dietz et al., 1995]. All three of these disorders are characterized by craniosynostosis, but a study of Shprintzen–Goldberg syndrome found that Chiari type I malformation was one of several anomalies found only in Shprintzen–Goldberg patients, distinguishing it from the other syndromes [Greally et al., 1998]. FBN1 is a major constitutive element of extracellular microfibrils with widespread distribution in connective tissues [Sakai et al., 1986], but not all patients with Shprintzen–Goldberg syndrome have CMI nor are all cases linked to FBN1. While it is unclear how large a role FBN1 may play in CMI, it is a plausible candidate gene to follow-up in patients and families affected with Chiari type I malformation.
Acknowledgements
We thank the reviewers for their helpful comments.




