Mitochondrial dysfunction in Stüve–Wiedemann syndrome in a patient carrying an ND1 gene mutation†
How to cite this article: Morava E, Hamel B, Hol F, Rodenburg R, Smeitink J. 2006. Mitochondrial dysfunction in Stüve–Wiedemann syndrome in a patient carrying an ND1 gene mutation. Am J Med Genet Part A 140A:2248–2250.
Abbreviations:
CS, citrate synthase; LIFR, leukemia inhibitory factor receptor; ND1, NADH-ubiquinone oxidoreductase, subunit ND1.
To the Editor:
Episodes of hyperthermia, liver failure, anemia, and mitochondrial dysfunction has been observed previously in Stüve–Wiedemann syndrome (SWS; OMIM 601559) [Stüve and Wiedemann, 1971; Chabrol et al., 1997; Cormier-Daire et al., 1998; Di Rocco et al., 2003]. Since no other patient with a proven LIFR (OMIM 151443; [Dagoneau et al., 2004]) gene mutation in combination with a respiratory chain dysfunction has been reported, the casual relationship between the metabolic disorder and SWS remained hypothetical. We describe a patient with SWS and a disorder of the oxidative phosphorylation, carrying a LIFR mutation and a heteroplasmic mitochondrial ND1 gene (OMIM 516000) mutation.
The index patient (V-1) was born at 40 weeks of gestation with a birth weight of 3,450 g (−0.5 SD), length of 44 cm (−4 SD), and head circumference of 32 cm (−1.5 SD) as the first male child of healthy, non-consanguineous Dutch parents. The mother (IV-1) and the maternal grandmother (III-2) had had frequent episodes of migraine; the latter suffered from normochromic anemia and hypothyroidism as an adult. The maternal great-grandmother (II-4) was also known to have hypothyroidism. Two distant maternal relatives (III-1 and III-4), with known psychomotor retardation and epilepsy died in childhood. A maternal third cousin (V-4) was diagnosed with a mitochondrial encephalomyopathy and mitochondrial complex I deficiency with a heteroplasmic (80%) ND1 gene mutation confirmed in a muscle biopsy (Fig. 1).
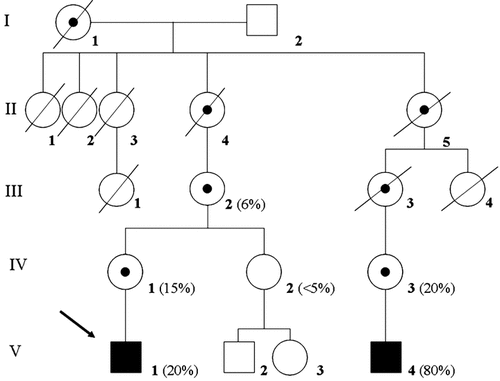
Pedigree demonstrating the segregation of the ND1 mutation in blood (percentage of heteroplasmy in bracelets) in five generations. The index patient (V-1) carrying a compound heterozygous LIFR mutation and a heteroplasmic ND1 mutation is labeled with an arrow. Patient V-4 is known with severe encephalomyopathy in combination with mitochondrial complex I deficiency. Patient III-2 has episodes of migraine, Patient IV-1 has migraine, hypothyreoidism, and anemia, whereas Patient IV-3 is followed for fatigue.
The propositus had campomelia and camptodactyly, severe generalized muscle hypotonia, a loud systolic heart murmur, episodes of hyperthermia (42.5°C), and neonatal respiratory failure, requiring mechanical ventilation for 3 weeks. Transient neonatal seizures were treated successfully with phenobarbital. The brain MRI showed small symmetrical lesions of high-signal intensity in the caudate nucleus on T2 images. An echocardiography demonstrated tricuspid insufficiency, patent foramen ovale, and non-obstructive hypertrophic cardiomyopathy. He also had a convergent strabismus of the left eye, telecanthus, apparently low set ears, retrognathia, short neck, narrow chest, short ribcage, a protruding abdomen, hypotonic abdominal muscles, campomelia, camptodactyly, ulnar deviation of fingers, long big toe, and partial syndactyly of second to third toes. The skeletal survey showed characteristic findings of SWS (Fig. 2). The patient received gastric tube feeding due to feeding difficulties. His motor development was delayed with head lag and inability to roll over till age 8 months. He developed corneal ulcerations. At 12 months he was already able to sit, had head control, he fed himself, and his gastric tube was removed. The cardiomyopathy improved significantly and required no further medication. He suffered from multiple ear infections, early caries due to abnormal tooth development, and from spontaneous femur fractures without pain. He started walking at 15 months but continued to have exercise intolerance with decreased muscle mass, muscle tone, and decreased deep tendon reflexes. Logopedic treatment was initiated due to an obvious speech delay. The repeated cranial MRI, visual evoked potentials, auditory evoked potentials, and an EEG were normal. The diagnosis of SWS was confirmed by sequence analysis ([Dagoneau et al., 2004], compound heterozygozity for 167-170delTAAC in exon 3 and C1865T in exon 13 in the LIFR gene).
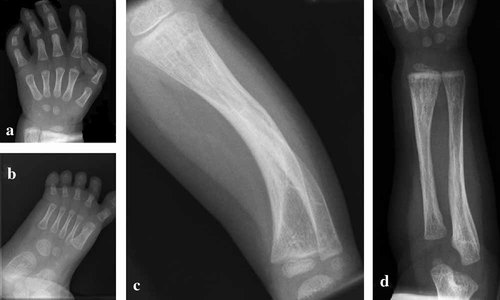
Skeletal photos of the hand, foot, distal arm, and distal lower limb of our patient at age 1 year. Note osteopenia, short and stubby metacarpals (a, b), and long bones (c, d), metaphyseal flaring and radiolucency with bowing of the tibia and fibula (c).
Due to the unexplained presence of cardiomyopathy, muscle cramps and mildly increased lactic acid levels in blood (2.1–2.8 mmol/L; controls <2.0 mmol/L) and an increased serum alanine concentration (575 mmol/L; controls: <450 µmol/L) a fresh muscle biopsy was performed [Janssen et al., 2006] showing a significant decrease in the in vitro ATP production from pyruvate oxidation (15 nmol ATP/hr mU CS; controls: 42–81 nmol ATP/hr mU CS, N:21). The activities of mitochondrial enzyme-complexes I, II, III, and IV were normal. Sequence analysis of the mitochondrial ND genes showed a heteroplasmic mutation of the ND1 gene (3697G>A, [Kirby et al., 2004]) in 26% in muscle and 20% in blood (V-1). His mother (IV-1) and grandmother (III-2) were diagnosed with a heteroplasmic mutation in blood as well (15% and 6%). The mother of propositus V-4 carried the same mutation in blood in 20% (Fig. 1).
So far one child has been described carrying the 3697G>A mutation with mitochondrial encephalomyopathy, spasticity, seizures, normal intelligence, lesions of the basal ganglia, and lactic acidemia. Enzyme histochemistry was normal in muscle, and complex I deficiency was present only as expressed relative to complex II [Kirby et al., 2004]. The pathogenicity of the 3697G>A mutation has been confirmed by measuring a decreased complex I assembly and decreased activity in fibroblasts, and the failure of restoring complex I activity by fusion of the patient's fibroblasts with ρ0 cells [Kirby et al., 2004]. We suggest that the observed signs of mitochondrial dysfunction in our index patient (V-1) are due to the presence of the heteroplasmic ND1 mutation. Moreover, proband V-4 (Fig. 1) demonstrated a fully consistent phenotype with those described by Kirby et al. 2004, however in our patient complex I deficiency was detectable in muscle tissue as well.
Here, we describe the unique combination of a deficient oxidative phosphorylation with SWS due to the combined coincidental occurrence of a LIFR gene mutation and ND1 gene mutation in a male patient. We suggest that mitochondrial dysfunction does not belong to the clinical picture of the Stüve–Wiedemann syndrome.
Acknowledgements
The authors were supported by the European Community's sixth Framework Program for Research, Priority 1 “Life sciences, genomics and biotechnology for health,” contract number LSHM-CT-2004-503116 and are thankful for the sequence analysis of the LIFR gene for Valerie Cormier-Daire.




