The expanding panorama of split hand foot malformation†
How to cite this article: Basel D, Kilpatrick MW, Tsipouras P. 2006. The expanding panorama of split hand foot malformation. Am J Med Genet Part A 140A:1359–1365.
Abstract
The split hand/foot malformation is a developmental defect of the extremities resulting from errors in the initiation and maintenance of the apical ectodermal ridge. The phenotype is genetically heterogeneous, and it can be identified either as an isolated phenotypic manifestation or as a constituent component of a malformation syndrome. This overview describes the clinical phenotype, related animal models, and the evolving genetic heterogeneity of the malformation. © 2006 Wiley-Liss, Inc.
INTRODUCTION
The split hand/foot malformation (SHFM) is a developmental defect of the extremities presenting at birth either as an isolated entity or as a syndromic manifestation. Nonsyndromic SHFM is genetically heterogeneous with at least five loci described to date. Recent studies have identified several signaling molecules, growth factors, and transcriptional regulators involved in the initiation and maintenance of the apical ectodermal ridge (AER). Studies of abnormal murine phenotypes have uncovered the role played by genes such as p63 and Dactylin in the maintenance of AER activity. These phenotypes resemble human malformations and have proven invaluable in elucidating the molecular pathology of SHFM.
Phenotypic Manifestations
The degree of phenotypic severity of an affected limb as well as the number of affected limbs differs from individual to individual. The core phenotype of SHFM could be categorized in three recognizable patterns (Fig. 1): (a) Monodactyly presenting as a single digital remnant derived either postaxially (observed in SHFM1–4) or preaxially as in SHFM5; (b) Bidactyly (so called “lobster-claw”—a pejorative term not recommended) presenting as two digital elements separated by a deep median cleft; and (c) Oligodactyly, by far the most common pattern, presenting as three or more digits in association with syndactyly and a median cleft [McKusick, 1998; Elliott et al., 2005]. Noncore phenotypic manifestations include clinodactyly, camptodactyly, triphalangeal thumb and ulnar deviation at either the MCP joints or the radio-ulnar-carpal junction [McKusick, 1998]. A recent report postulated increased incidence of preaxial involvement in the upper limbs in families with SHFM3 [Elliott et al., 2005]. Approximately 40% of individuals presenting with SHFM have associated anomalies suggestive of a syndromic entity. The overall prevalence of SHFM is reported to be approximately 1:18,000 newborns [McKusick, 1998]

Phenotypic variability of the distal limb defects observed in SHFM. The three types of defects seen, monodactyly, bidactyly, and oligodactyly are shown from left to right. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Genetic Heterogeneity of SHFM
SHFM occurs either sporadically or in families. Reduced penetrance is frequently observed, and it has been documented in several pedigrees. Five SHFM loci have been established, four autosomal and one X-linked (Table I). The four autosomal loci are SHFM1 (7q21.2–22.1) [Scherer et al., 1994], SHFM3 (10q24–25) [Nunes et al., 1995; Gurrieri et al., 1996; Raas-Rothschild et al., 1996], which has been shown to result from complex gene rearrangements in the DACTYLIN gene and adjacent loci, SHFM4 (3q27) [Ianakiev et al., 2000], resulting from mutations in the p63 gene, and SHFM5 (2q24–q31) [Boles et al., 1995]. The X-linked SHFM2 maps to Xq26 and is phenotypically similar to the autosomal dominant forms [Ahmad et al., 1987; Faiyaz ul Haque et al., 1993].
| Type of SHFM | Gene | Reference | |
|---|---|---|---|
| SHFM1 | 7q21.2–22.1 |
[Scherer et al., 1994] |
|
| SHFM2 | Xq26 | ||
| SHFM3 | 10q24–25 | Dactylin |
[Nunes et al., 1995; Gurrieri et al., 1996; Raas-Rothschild et al., 1996] |
| SHFM4 | 3q27 | p63 |
[Ianakiev et al., 2000] |
| SHFM5 | 2q31 |
[Boles et al., 1995] |
|
| SHFM6 | ? |
- The genes responsible for the two loci for which a molecular basis has been determined are shown. Based on the existence of families unlinked to any of the mapped loci, at least one additional SHFM locus exists.
MOUSE MODELS OF SHFM
The Dactylaplasia Mouse
The heterozygous Dactylaplasia (dac) phenotype in mice is characterized by the absence of central digital rays (Fig. 2), and closely resembles the SHFM [Crackower et al., 1998]. This phenotypic similarity, as well as the localization of dac to chromosome 19 [Johnson et al., 1995], in a region syntenic to SHFM3 (10q24), made it an ideal model for studying the molecular mechanisms involved in SHFM. Cloning and analysis of the mouse dactylin gene identified mutations in the Dac mouse [Sidow et al., 1999]. Molecular genetic analysis of Dac mice characterized two dactylyplasia alleles. In dac1J the dactylin gene contains a 4.5 kb insertion 10 kb upstream of the gene [Sidow et al., 1999]. In dac2J the dactylin gene contains a 5.5 kb intronic insertion [Sidow et al., 1999]. The normal transcript is absent and low levels of a 9.5 kb mutant transcript is present [Sidow et al., 1999]. Initial genetic experiments revealed that dac expression was further modulated by a modifier gene, mdac, mapped on chromosome 13 and that both dac alleles were equally sensitive to the effects of the mdac suppressor alleles [Chai, 1981; Johnson et al., 1995].
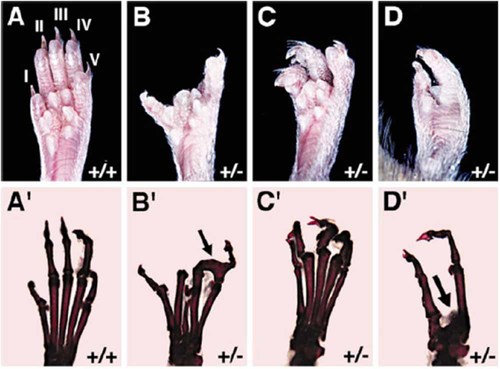
Skeletal analysis of Dac limb phenotype, showing wt (A, A′) and Dac/+ (B–D, B′–D′) adult hind limbs. Panels B, B′ show typical Dac/+ phenotype with missing digits 2 and 3, and digits 4 and 5 fused at the level of the fist phalange (arrow in B′). Panels C, C′ show less severe phenotype with only soft tissue fusion between digits 1 and 2, and 4 and 5. Panels D, D′ show severe phenotype with absence of digits and metatarsals 2, 3, and 4 (arrow in D′). (modified from Crackower et al. 1998) [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Crackower et al. 1998 have shown that the primary mechanism underlying the loss of central digital rays in affected limbs is a defect in the maintenance of the AER activity, allowing the normally high level of cell death to eliminate the central aspect of the AER prematurely. In heterozygous dac mice, there is an abnormally low level of fgf 4, fgf8, bmp2, and bmp4 in the anterior and central aspect of the AER, while the expression of these signals is maintained posteriorly [Crackower et al., 1998]. Sidow et al. 1999 proposed the existence of a suppressor, which would be degraded by dac to allow an appropriate level of cell proliferation in the AER. The structure of dactylin suggests it as a member of the F-Box/WD40 family of proteins, which are known to be involved in cell cycle regulation via ubiquitin mediated protein degradation [Ianakiev et al., 1999]. In Dac mice this suppressor would not be degraded and cell proliferation would diminish, thus shifting the balance between cell proliferation and cell death, resulting in the premature elimination of the AER [Sidow et al., 1999].
The p63 Knockout Mouse
The p63 gene is widely expressed in the progenitor cells of many epithelial tissues, particularly in limb bud AER, branchial arches, and the epidermal appendages [Yang et al., 1998, 1999; Mills et al., 1999]. Limb truncations, striking craniofacial abnormalities, and complete absence of the epidermal components are among the malformations noted in the p63−/− mouse mutation [Mills et al., 1999; Yang et al., 1999]. Histological sections taken from p63 deficient mice show abnormally small limb buds and an absent AER [Mills et al., 1999].
The p63 gene is a homolog of the tumor suppressor gene, p53. However, unlike p53, transcription of p63 is regulated from two distinct promoter regions, producing either transactivating (TA-p 63) or nontransactivating (N-p63) isotypes. The N-p63 isotypes have the remarkable ability to induce apoptosis and act as dominant-negative agents towards transactivation of p53 and p63 [Yang et al., 1999]. The p63 gene has been identified as an essential transcription factor in the mesenchymal–ectodermal signaling of the AER [Osada et al., 1998; Senoo et al., 1998; Trink et al., 1998; Yang et al., 1999]. The effect of mutations in the p63 gene on limb malformation has been attributed to an inability of the AER to differentiate and thus maintain its structural integrity [Mills et al., 1999; Yang et al., 1999].
MOLECULAR PATHOLOGY OF SHFM
Dactylin and SHFM3
The Dac mouse provided the model for the elucidation of the molecular basis of SHFM3 given the synteny between the human 10q24 region and mouse chromosome 19 where the dac locus was localized. The human DACTYLIN gene was mapped in the 10q24 region. The cloning and characterization of the human DACTYLIN gene revealed similar structure between the mouse and human genes, thus confirming dactylin as a novel member of the F-box/WD40 protein family [Ianakiev et al., 1999; Ilyin et al., 2000]. The DACTYLIN gene comprises nine exons distributed in more than 85 kb of genomic DNA (Fig. 3). SNP analysis confirmed the localization of the Dactylin locus within the critical region for SHFM3.
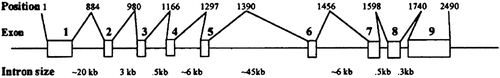
Genomic organization of the human DACTYLIN gene. The nine exons are shown as boxes, the positions of the introns shown above, and the estimated intron size below.
Extensive analysis of the coding sequence of the human DACTYLIN gene in several individuals affected by SHFM did not reveal any mutations [Ianakiev et al., 1999]. Additionally, comprehensive mutation screening of several candidate genes within the SHFM3 critical region failed to identify any causative coding sequence alterations in a number of candidate genes [de Mollerat et al., 2003a; Kano et al., 2005; Basel et al., unpublished data]. The molecular basis of SHFM3 was further investigated using Positional Transcript Analysis.
Positional transcript analysis
This approach first defined the critical region containing the disease gene of interest. Transcript analysis was then performed by screening transcripts produced from the critical region for the presence of any mutations. In silico analysis of the SHFM3 critical region determined that it contained 26 known genes, 13 putative genes, and 26 putative transcripts (Fig. 4). Transcript analysis was performed by initially screening with heteroduplex analysis, followed by direct sequence analysis. No causative mutations were found in any of the transcripts analyzed.
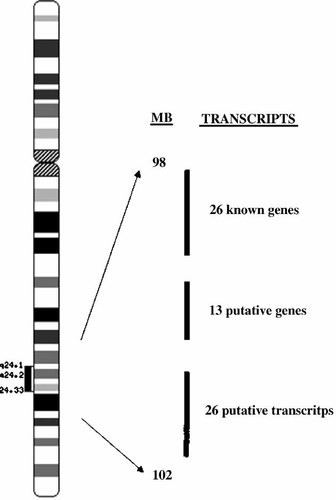
Number of transcripts within the 4 Mb SHFM3 critical region that were analyzed by Positional Transcript Analysis.
Transcript quantitation
The lack of identifiable mutations in the genes mapped in the SHFM3 critical region did not rule out the possibility of regulatory mutations that might be affecting the level of gene transcripts. Transcript levels of several genes in proximity to the DACTYLIN locus within the SHFM3 critical region were, therefore investigated. Quantitative PCR was used to determine transcript levels, in RNA from a panel of SHFM individuals, for eight genes; CHUK, TLX1, LBX1, BTRC, DAC, FGF8, NFKB2, and SUFU (Fig. 5) [Basel et al., 2003]. A significant difference in expression was detected between five affected individuals and normal controls for DACTYLIN (P < 0.0001) and LBX1 (P = 0.0002) (Fig. 5). No difference was detected in another six affected individuals. No difference in transcript levels was detected for TLX1, BTRC, FGF8, NFKB2, CHUK, and SUFU, however transcript levels for BTRC and FGF8 were low in all samples. The twofold decrease in the level of DACTYLIN gene transcript detected in five unrelated individuals affected with nonfamilial SHFM as compared to unaffected controls supported a central role for DACTYLIN in the pathogenesis of SHFM3 [Basel et al., 2003].
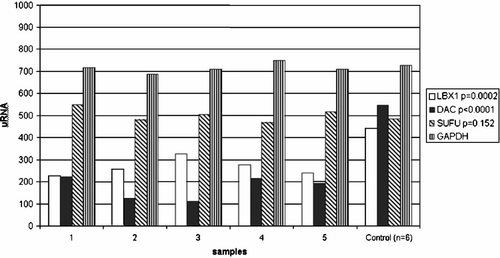
Transcript levels for eight genes within the SHFM3 critical region; CHUK, TLX, LBX1, BTRC, DAC, FGF8, NFKB2, and SUFU, were analyzed by QPCR. Gene expression levels are shown for LBX1, DAC, SUFU, and GAPDH control, for five unrelated affected patients (numbered 1 through 5) compared with six normal control individuals.
These data are consistent with the findings of de Mollerat et al., 2003b, who demonstrated complex rearrangements associated with large duplications of 10q24 in seven SHFM families. These rearrangements result in an extra copy of a segment of the DACTYLIN gene, duplication of the BTRC, POLL, and LBX1 genes, and additional genomic sequence located approximately 500 kb centromeric to the DACTYLIN gene [de Mollerat et al., 2003]. Similar rearrangements have been reported by an independent group [Kano et al., 2005].
p63 and SHFM4
Gene-targeting studies have shown that p63 plays a critically important role in regulating the formation and differentiation of the AER. Mice lacking p63 activity have partial or total limb truncations, thus implicating the human p63 gene as a candidate for SHFM. Sequence analysis of genomic DNA from families segregating SHFM initially identified two missense mutations in the p63 gene [Ianakiev et al., 2000]. These mutations, in exons 5 and 7, respectively, both fall within the DNA-binding domain of the p63 molecule, which extends from exons 4–8. Subsequently, at least 28 different p63 gene mutations have been reported in SHFM families [Celli et al., 1999]. The majority of mutations resulting in SHFM have been identified within the DNA binding domain. A few mutations however, fall outside this region, including a nonsense mutation in the 3′ region of the gene and an exon 3 mutation which involves the transactivation domain [Zenteno et al., 2005]. Only a fraction of cases of SHFM are due to p63 gene mutations [de Mollerat et al., 2003].
Mutations in the p63 gene are also responsible for ectrodactyly–ectodermal dysplasia-cleft lip/palate(EEC), acro-dermato-ungual-lacrimal-tooth (ADULT) and limb-mammary syndrome (LMS) which are syndromic disorders featuring split hand and foot malformations [Celli et al., 1999; Wessagowit et al., 2000]. Hay-Wells syndrome has also been found to be caused by p63 gene mutations [McGrath et al., 2001].
SHFM1, SHFM2, and SHFM5
SHFM1 has been identified in individuals found to have a chromosomal rearrangement involving 7q21–q22 [Scherer et al., 1994]. A microdeletion of this region has refined the critical interval to 0.9 M, which includes the candidate genes DSS1, DLX5, and DLX6 [Wieland et al., 2004]. Animal models in which dlx5 and dlx6 have been targeted for deletion exhibit an SHFM phenotype only if both dlx5 and dlx6 genes are deleted [Robledo et al., 2002]. A direct cause between mutations within these genes and SHFM has not been made in humans.
The critical region for the X-linked SHFM2 has recently been narrowed to 5 cm and screening of the 19 candidate genes within this region did not identify a causative mutation [Faiyaz-Ul-Haque et al., 2005].
In SHFM5, a chromosomal rearrangement has been identified at 2q24–q31, which includes the candidate genes DLX1 and DLX2 [Qiu et al., 1997]. However, neither homozygous nor heterozygous knockout mouse models exhibit an SHFM phenotype [Zakany and Duboule, 1996]. The HOXD cluster of genes is located adjacent to the deleted region and mutations within this cluster have been shown to cause synpolydactyly [Zakany and Duboule, 1996; Goodman et al., 2002]. There is however some evidence in animal studies to suggest that a telomeric regulator of the Hox D genes may be the causative gene involved in SHFM5 [Goodman et al., 2002] which would also account for the phenotypic differences between monodactyly noted in limbs of individuals affected with SHFM5 versus SHFM1-4. The Hox D genes are regulated from the posterior mesenchyme of the developing limb with both spatial and temporal colinearity [Herault et al., 1997; Kmita et al., 2002]. The anterior segment of the limb is least affected in SHFM5 as the Hox A cluster exerts their regulatory effects anteriorly while the posterior segment is Hox D dependant and thus monodactyly is represented by the radial element [Herault et al., 1997; Peichel et al., 1997; Del Campo et al., 1999]. This mechanism is in contrast to the current understanding of the pathogenesis of monodactyly in SHFM3 and SHFM4, which maintain that the posterior element is retained in monodactyly. It is of interest to note that elimination of both Hox A and Hox D expression results in severe limb truncation defects [Kmita et al., 2005].
Distance Effects
One of the two dactylaplasia mouse mutations affects neither the amount nor the integrity of the dactylin transcript and it cannot be excluded, therefore, that dac alleles disrupt long-range regulatory sequences from one or more genes. Disruption of a distant regulator of downstream developmental genes has also been postulated as the cause in both SHFM1 and SHFM5. The skeletal dysplasias as a group are replete with examples of genes being modulated by distant regulators. An interesting example of this is the disruption of long-range regulatory sequences of the sonic hedgehog gene which have been shown to play a causative role in the limb malformation preaxial polydactyly (PPD) [Lettice et al., 2002]. Two categories of malformations resulting from the abnormal expression of the LMBR1 gene have been identified in humans and mouse; limb reduction defects as in acheiropodia and duplications, fusions, and aplasias of skeletal elements as in PPD, (Hemimelic extra-toes) Hx, and (Sasquatch) Ssq. The acheiropodia phenotype appears to result from a loss-of-function mutation, while PPD, Hx, and Ssq appear to be the consequence of gain-of-function mutations of the same gene. Interestingly enough, both the acheiropodia and the Ssq mouse mutations point to the possible role of mutant LMBR1 in the mis-regulation of sonic hedgehog. This regulatory phenomenon is not unique to skeletal development but nevertheless plays a critical role and as our understanding evolves, we hope to gain more insight into the complex nature of gene regulation and malformation.




