A novel RSK2 (RPS6KA3) gene mutation associated with abnormal brain MRI findings in a family with Coffin–Lowry syndrome†
How to cite this article: Wang Y, Martinez JE, Wilson GL, He X-Y, Tuck-Muller CM, Maertens P, Wertelecki W, Chen T-J. 2006. A novel RSK2 (RPS6KA3) gene mutation associated with abnormal brain MRI findings in a family with Coffin–Lowry syndrome. Am J Med Genet Part A 140A:1274–1279.
Abstract
Coffin–Lowry syndrome (CLS) is an X-linked mental retardation syndrome caused by defects in the RSK2 gene. We have identified a CLS family with four patients in two generations. The patients in this family, a mother and her three children (a male and two females), all have severe mental retardation with the typical CLS phenotype. In addition, brain MRI studies on the three siblings revealed abnormalities in deep subcortical white matter, thinning of the corpus callosum, hypoplastic cerebellar vermis, and asymmetry of the lateral ventricles. The degree of severity of the MRI findings correlated with the severity of mental retardation in the patients. Extensive mutation screening was performed on the entire RSK2 gene in this family. Twenty-two exons including the intron/exon junctions were amplified by PCR and subsequently sequenced on both strands. A novel mutation, a two-nucleotide insertion (298 ins TG), was identified. The insertion creates a stop codon at codon 100, resulting in a 99 amino acid truncated RSK2 protein. All patients tested have the same mutation, and no other mutation could be found in the RSK2 gene from the proband. The mutation was confirmed by PCR/RFLP. X-chromosome inactivation assay on the female patients revealed significant skewing toward inactivation of the normal RSK2 allele. Thus, this novel mutation is likely to be responsible for the unusual clinical presentation in this family, which includes full phenotypic expression in females and unique brain MRI abnormalities. The pathological function of the mutation and genotype/phenotype correlation between the mutation and this unusual clinical presentation await further clarification. © 2006 Wiley-Liss, Inc.
INTRODUCTION
The Coffin–Lowry Syndrome (CLS, OMIM #303600) is an X-linked mental retardation syndrome caused by defects in the RSK2 (RPS6KA3) gene, which maps to Xp22. In addition to impairment of cognitive and adaptive functions, the characteristics of the syndrome include craniofacial dysmorphism, growth failure, and distally tapering fingers. Other clinical findings include short stature, pectus deformity, kyphosis and/or scoliosis, mitral valve dysfunction, and sensorineural hearing loss. Affected males usually have severe mental retardation, whereas females present a wide range from severe to relatively normal [Hanauer and Young, 2002]. CLS patients have been diagnosed from every ethnic group.
RSK2 gene is a member of a ribosomal S6 serine/threonine kinase family. This RSK gene family plays a key role in the Ras-MAPK signaling pathway. RSKs are activated by phosphorylation in response to a broad range of cellular signals, including insulin, growth factors, and neurotransmitters. It has been shown that these proteins are involved in several important cellular events, such as proliferation, differentiation, and apoptosis. Several transcriptional factors have been identified as substrates of RSK2, including the cAMP responsive element binding protein (CREB), c-Fos, and c-Jun. It has been suggested that Ras-MAPK pathway signaling through RSK2 to CREB plays an important role in learning and memory [Harum et al., 2001; Weeber et al., 2002].
Since 1996, more than 100 mutations in the RSK2 gene have been identified in CLS patients (CLS mutation database at Alsace.u-strasbg.fr/chimbio/diag/coffin/listmut.html). These mutations are distributed over the entire gene including both coding regions and introns, and about two-thirds of them result in premature translation termination. However, an extensive mutation screen was performed on 250 clinically diagnosed CLS patients, using single strand conformation polymorphism (SSCP) combined with Western blot and RSK2 activity assays. Mutations were found in only 50% of the patients [Zeniou et al., 2002b]. These results suggest that CLS is genetically heterogeneous. Furthermore, no consistent genotype/phenotype correlation has been established in CLS. Specific mutations have not been linked either to the severity of disease or the expression of a particular clinical feature [Delaunoy et al., 2001].
Most female CLS patients show mild mental retardation with variable craniofacial features that are similar to those seen in male patients. Skewed X-inactivation, which has been demonstrated to correlate with clinical severity in females with other X-linked diseases, could not be confirmed on blood samples from six female CLS carriers [Hanauer and Young, 2002]. In another study of eight female carriers, skewed X-inactivation was confirmed with a skewing mean of 88:12. However, the correlation between skewed X-inactivation and cognitive impairment was not statistically significant [Simensen et al., 2002].
More than 80% of CLS cases are sporadic and only a few families with mutation transmission have been reported (reviewed by Hanauer and Young 2002). In this study, we report a CLS family with four patients in two generations, a mother and her three children (a male and two females). The male patient in this family has severe mental retardation with typical CLS phenotype. In addition to severe to mild mental retardation with typical CLS phenotype, two of the three female patients have developed prominent varicose veins in the lower extremities. Of further interest, brain MRI studies of this family revealed abnormalities not previously reported in CLS patients. We performed extensive mutation screening on the whole RSK2 gene in this family. A novel mutation, a two base pair insertion in exon 4 of the RSK2 gene, was identified. X-inactivation studies were performed on two of the female patients, and the results showed that X chromosome inactivation was highly skewed towards the X chromosome with normal RSK2 allele.
MATERIALS AND METHODS
Mutation Detection
DNAs were isolated from peripheral blood samples using a modified non-enzymatic method as described by Lahiri and Nurnberger 1991. PCR primers were obtained from Invitrogen (Carlsbad, CA) to amplify every exon of the RSK2 gene. Primer sequences were designed according to a previous report [Jacquot et al., 1998]. Each exon of RSK2 gene was PCR amplified under optimized conditions. PCR products were purified and sequenced on both strands using a BigDye terminator cycle sequencing kit on an automatic sequencer with DNA sequencing analysis software package from Applied Biosystems, Inc. (Foster City, CA).
Reconfirm Mutation by RFLP
Exon 4 of RSK2 was amplified from patient DNA under optimized condition. The PCR products were digested with Bsl I in NEBuffer3 (New England BioLabs, Beverly, MA) at 55°C for 6 hr. Restricted samples were loaded on 3% agarose gel and the gel image under UV illumination was captured by Kodak gel documentation system.
X-Chromosome Inactivation Studies
X-inactivation studies were performed on the human androgen receptor gene (HUMARA locus) according to a modified protocol described by Allen et al. 1992 using a florescence labeled primer set. DNAs were isolated from peripheral blood samples from two of the affected female patients, and from six unrelated normal female controls, all of whom have more than three different CAG repeat numbers within the region. Prior to PCR, each DNA sample was incubated overnight with either Hha I, Hpa II, or with digestion buffer only (New England BioLabs). Digested DNAs were PCR amplified, and PCR products were injected on to a Genetic Analyzer (PRISM 310, ABI). Data analysis was done using GeneScan and Genotyper software from ABI. Since the two female patients and female controls were all heterozygous on HUMARA locus, two PCR amplicom peaks were detected from all female samples. The peak area of each allele was normalized by the formula: Peak Area(1 or 2)/(Peak Area1 + Peak Area2) × 100%, where 1 and 2 referred to allele 1 and allele 2 of HUMARA locus. The X inactivation pattern was defined as the ratio of the values of the two normalized peak areas. Ten STR markers (ABI) located on chromosome Xp22 to Xq21 were used to establish the linkage between the RSK2 gene and the HUMARA gene in the patients.
CLINICAL AND LABORATORY RESULTS
Clinical Findings
The proband, a Caucasian boy, was referred for a medical genetics evaluation at age 13 months because of mental retardation and dysmorphic features. He is the third of three pregnancies to his mentally retarded mother. The proband has severe mental retardation, short stature, universal hypotonia, and characteristic features of CLS including prominent ears, hypertelorism, down-slanting palpebral fissures, broad nose, maxillary hypoplasia, open mouth with thick and everted lower lip, pectus carinatum, thoracic scoliosis, translucent skin, delayed dentition, and tapering fingers (Fig. 1). At age 8 years, he had an OFC of 52 cm (50th centile), height of 116.5 cm (5th centile), and weight of 24 kg (25th centile). Chromosome analysis and DNA studies for Fragile X syndrome were negative.
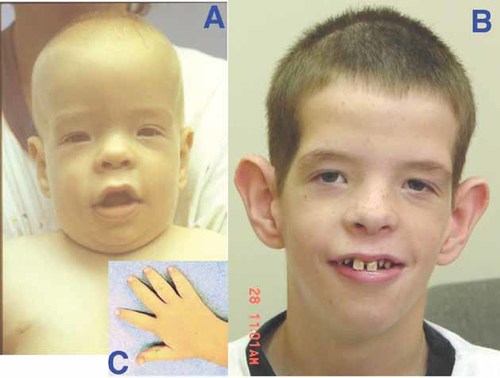
Proband at age 13 months (A) and 8 years (B). Note prominent ears, hypertelorism, down-slanting palpebral fissures, broad nose, maxillary hypoplasia, and open mouth with thick, everted lower lip, as well as distally tapered fingers (C).
The proband's mother has coarse facial features, thick lips and tapering fingers. Detailed information on her physical features is otherwise not available. She did not have MRI studies, and she declined to offer a blood sample for molecular analysis. Her first two pregnancies produced two affected females with typical CLS phenotypes including cognitive deficiencies.
The older sister of the proband has moderate to severe mental retardation, obesity, and dysmorphic features (Fig. 2). At the age of 16 years, her measurements included: OFC 54.2 cm (50th centile), height 162.5 cm (50th centile), and weight 85 kg (>95th centile). She was recently diagnosed with schizophrenia and she has developed prominent varicose veins in the lower extremities.
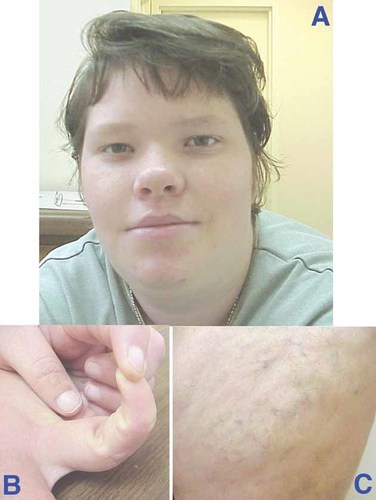
Proband's older sister at age 16 years. Note coarse features, hypertelorism, prominent jaw, everted lower lip (A), distally tapered fingers and hyperelasticity of joints (B), and prominent varicose veins (C).
The younger sister of the proband also has moderate mental retardation and dysmorphic features, including hypertelorism, down-slanting palpebral fissures, prominent jaw and distally tapered fingers. Her measurements included: OFC 53 cm (50th centile); height 160 cm (25th centile); and weight 78 kg (90th centile). She also has developed prominent varicose veins.
Brain MRI Studies
The MRI studies of all three siblings revealed numerous focal areas of cerebrospinal fluid (CSF) signals in the deep cortical matter, more prominent in the frontal lobe and on the left. In addition, mild asymmetry of the lateral ventricles, thinning of the corpus callosum and hypoplastic cerebellar vermis were present (Fig. 3). The severity of these MRI findings correlated with the severity of mental retardation in these siblings.
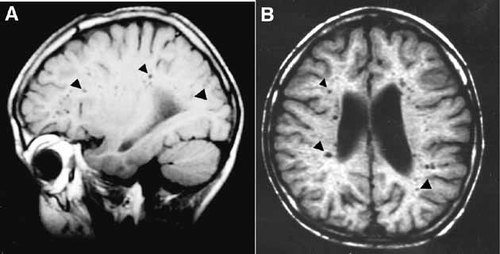
MRI image on lateral (A) and axial (B) of proband's brain. Note numerous focal areas of CSF signals (arrowhead) in the deep cortical matter, and mild dilatation of the ventricles.
Mutation Identification
All 22 exons of RSK2 gene were sequenced on both strands in the DNA samples from the proband and his older sister, as well as in a DNA sample from an unrelated normal female. The sequence results were compared with the sequence data from the human genome project in the GeneBank. In the proband and his older sister, a two base pair TG insertion mutation was detected at nucleotide position 298 of the RSK2 cDNA (GeneBank accession #: NM_004586) (Fig. 4A). This 298insTG mutation is located in exon 4, resulting in a premature stop codon at codon position 100. The mutant protein predicted is truncated from 740 amino acids to 99 amino acids (Fig. 4B). Thus, the function of the mutant RSK2 is predicted to be completely lost in the affected male. Sequencing of the RSK2 exon 4 of proband's younger sister detected the same mutation at the same position. This mutation was not found in the sample from normal control female. No other mutations in the entire RSK2 gene were detected in either the proband or his older sister.
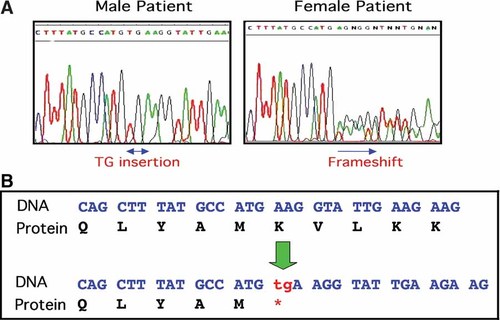
A: Identification a TG insertion mutation on RSK2 gene by sequencing analysis. Result from proband (left) and from his sister (right). Note heterozygous mutation in the female patient. B: Two base pair insertion mutation results in a stop codon and a 99 amino acid truncated RSK2 protein.
To confirm the presence of the mutation in the patient samples, a PCR/RFLP assay was developed. The mutation with the two base pair insertion creates a new Bsl I restriction site in the exon 4 PCR fragment, while the wild-type allele is not digested with this restriction enzyme. The restriction pattern of proband's sample confirmed that the mutation was present (Fig. 5, affected male). The restriction pattern from the proband's sisters also detected the mutation on one allele, while the other allele is normal (Fig. 5, affected females).
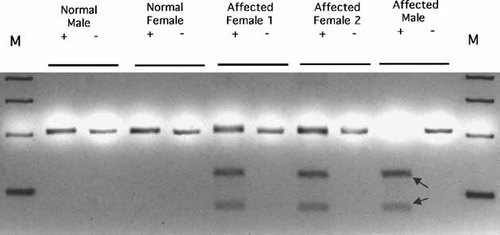
PCR/RFLP analysis confirmed the novel mutation on patient's samples. Note the affected male only had the mutated allele. The two affected females carried one mutated allele. Arrows indicated the mutation fragments restricted by Bsl I.
X-Inactivation Assay
As indicated in Figure 6, the normal female control samples showed a random inactivation pattern for the X chromosome pair. The X-inactivation ratio between the two alleles was 58:42 before digestion, 54:46 after digestion with Hha I, and 55:45 after digestion with Hpa II. Both of the analyzed female patients had a common HUMARA allele with amplicom size 279 base pairs. This common allele was demonstrated to be located on the same X chromosome as the defective gene RSK2 genes by STR linkage analysis. Both of the analyzed female patients showed significant skewing of the inactivation pattern. The ratio between the active X chromosome (with mutant RSK2 gene) and the inactive X chromosome (normal allele) was 17:83 for the older sister and 11:89 for the younger sister when digested with Hpa II, while, without digestion, the ratio was 59:42 and 45:56, respectively (P < 0.01). The skewing of the inactivation pattern was also confirmed by using a second restriction enzyme Hha I in the assay.
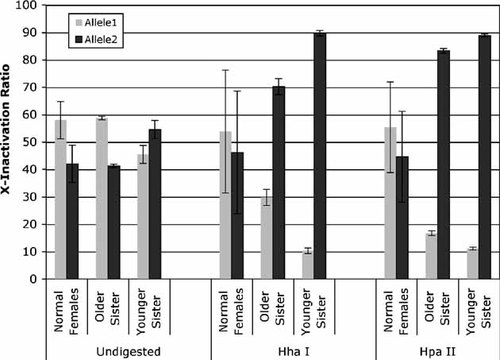
X-chromosome inactivation assay on the HUMARA locus. X-inactivation ratios were calculated by the formula defined in the text. The data presented were averaged from six repeated experiments and the standard deviation is indicated by error bar.
DISCUSSION
Even though mental retardation is the major impairment in CLS individuals, very few neuroimaging studies on CLS patients have been reported [Hanauer and Young, 2002; Patlas et al., 2003]. Only one study has reported brain abnormalities in a CLS patient and they were referred as small focal areas of hypointensity in the frontal white matter [Kondoh et al., 1998]. However, no correlation between a genotype and brain MRI findings has been reported. Brain MRI findings from our patients showed a characteristic, well-defined abnormality in the white matter. These lesions may be due to the primary pathologic process in CLS as a result of dysfunction of the RSK2 gene. These structural changes in the white matter may suggest that the RSK2 gene plays a role in the normal function of this region and these structural changes may be responsible for mental retardation in CLS. However, expression analysis of the RSK2 gene in human and mouse brain indicates that the expression of the RSK2 gene is very low in most brain structures, with no detectable RSK2 expression in white matter. The regions with highest expression of RSK2 in the brain were Purkinje cells of the cerebellum and pyramidal cells of hippocampus [Zeniou et al., 2002a]. Therefore, the location of the abnormal MRI findings in our patients is not consistent with the regions of RSK2 gene expression in the expression analysis study. This may be due to the limited sensitivity of the immunochemical staining method for RSK2 protein used in the previous study. The other possibility is that the RSK2 protein is truly not present in white matter, but RSK2 and/or its signaling pathway may regulate the expression of a subset of genes, whose function may be necessary for the development and function of white matter. The defects in white matter in CLS patients may indicate that the RSK2 gene is also involved in other brain physiological functions besides long term memory. Thus, the structural abnormalities in white matter may be one expression of the underlying pathological process, which is poorly understood in CLS patients.
More than 100 mutations have been identified in the RSK2 gene. However, no genotype/phenotype correlations have been established [Hanauer and Young, 2002]. Furthermore, no consistent relationship between a specific mutation and severity of disease has been identified. However, milder clinical presentations could be expected from patients who carry missense mutations. The novel mutation identified in this family members result in a very short truncated protein. All of the N-terminal kinase domain and C-terminal kinase domain, as well as the ERK docking site, are deleted from this truncated protein. Therefore, the function of RSK2 protein is predicted to be totally lost in the male patient. The typical CLS clinical manifestations with abnormal MRI findings in this family are associated with a novel mutation. However, MRI studies and mutation analyses of additional CLS patients are needed before genotype/phenotype correlations can be established.
The female patients in this family have less severe mental retardation than the proband, as well as less deteriorative findings in brain MRI. This may be due to residual function of RSK2 from the normal X chromosome, as indicated from the X inactivation assay. However, the samples used in the X inactivation assay were from peripheral blood and, therefore, may not represent the X-inactivation status in brain or other tissues. Our study is not consistent with the previous reports which showed either no skewing of X-inactivation in CLS carriers [Hanauer and Young, 2002], or no significant correlation between skewed X-inactivation and cognitive impairment [Simensen et al., 2002]. The correlation between the skewing of X-inactivation and severity of the disease should be further studied.




