Genotype–phenotype correlations in mapped split hand foot malformation (SHFM) patients†
How to cite this article: Elliott AM, Evans JA. 2006. Genotype–phenotype correlations in mapped split hand foot malformation (SHFM) patients. Am J Med Genet Part A 140A:1419–1427.
Abstract
Split hand foot malformation (SHFM) also known as central ray deficiency, ectrodactyly and cleft hand/foot, is one of the most complex of limb malformations. SHFM can occur as an isolated malformation or in association with other malformations, as in the ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome and other autosomal dominant conditions with long bone involvement, all showing variable expressivity and reduced penetrance. The deficiency in SHFM patients can also be accompanied by other distal limb anomalies including polydactyly and/or syndactyly. This variability causes the phenotypic classification of SHFM to be far from straightforward and genetic heterogeneity, with at least five loci identified to date, further complicates management of affected patients and their families. Although genotypic–phenotypic correlations have been proposed at the molecular level for SHFM4 patients who have mutations in the P63 gene, phenotypic correlations at the chromosomal level have not been thoroughly documented. Using descriptive epidemiology, Chi square and discriminant function analyses, our laboratory has identified phenotypic patterns associated with the mapped genetic SHFM loci. These findings can assist in classification, provide insight into responsible developmental genes and assist in directing mapping efforts and targeted genetic testing, resulting in more accurate information for family members in the clinical setting. Comparison with relevant animal models is discussed. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Various classifications of split hand have been proposed—general and specific, anatomic and genetic. Typical and atypical split hands were originally differentiated by Lange 1937 and this distinction has been maintained by others [Birch-Jensen, 1949; Barsky, 1964]. Atypical split hand often shows unilateral involvement and occurs sporadically. Generally, the feet are not involved. Clinically, there is a deficiency of the three central rays. The remaining rays are often hypoplastic and a web may exist in place of the deficient digits. It has been postulated that this entity may be the consequence of vascular interruption in some cases [Hoyme et al., 1983; Graham, 1986]. There is controversy in the surgical literature as to how this entity should be classified and significant confusion regarding nomenclature. In 1993, the Committee of the International Federation of Societies for Surgery of the Hand recommended that the term “atypical cleft hand” be discontinued and the term “symbrachydactyly” be used to identify the condition [Manske, 1993]. However, many geneticists continue to refer to this entity as atypical split hand.
In typical split hand, there may be bilateral involvement and the feet can also be affected. Unlike atypical split hand, patients with typical split hand may have a positive family history. Typical split hand has been classified into various types with one of the most common distinctions being Type I, which is an absence of central rays, frequently characterized by a cone-shaped cleft that divides the hand into two parts, and Type II or monodactyly, where the 5th finger remains and there is no cleft [Lewis and Embleton, 1908; Birch-Jensen, 1949; Temtamy and McKusick, 1978].
In addition to the cleft or digital deficiency, it is not uncommon for other distal limb abnormalities, such as polydactyly, syndactyly, triphalangeal thumb, transverse phalanges (“cross bones”) and/or delta phalanges, to accompany SHFM. The phenomenon of fusion (syndactyly) leads to an intriguing relationship between cleft hand, polydactyly, and syndactyly that has been discussed by various groups [Miura, 1976; Miura, 1978; Watari and Tsuge, 1979; Ogino, 1990; Ogino, 2004]. The International Federation of Societies of Surgery of the Hand Classification of Congenital Anomalies classifies split hand as Category I-Failure of Formation of Parts (Arrest of Development) [Swanson, 1976]. However, it is clear that elements of Category II-Failure of Differentiation (Separation) of Parts, as well as Category V, undergrowth (Hypoplasia) are often associated. In addition, Central Polydactyly, which is classified in Category III (Duplication), can also be present.
Vogel separated pedigrees in the literature based on the involvement of the lower limbs. The first group had affected members with constant involvement of the feet and regular autosomal dominant inheritance whereas individuals in the second group of families had variable involvement of the feet and irregular inheritance [Vogel, 1958]. However, other authors disagreed with Vogel's original classification [Temtamy and McKusick, 1978]. The many intriguing characteristics of SHFM pedigrees have also led to much speculation concerning the genetic mechanisms involved. Two or more affected sibs with normal parents suggested gonadal mosaicism [Auerbach, 1959], which was further supported by the report of two affected half sisters with the same unaffected father and different mothers [De Smet et al., 2001]. Spranger and Schapera 1988 suggested premutation of an autosomal dominant gene or cosegregation of an epistatic gene linked to split hand due to the apparent anticipation in their extensive pedigree. Segregation distortion (a departure from normal Mendelian ratios) has been observed as well as an apparent overtransmission of SHFM from affected fathers to sons [Jarvik et al., 1994; Ozen et al., 1999].
Clearly in some families, there can be extreme variability within and between individuals, suggesting the involvement of additional genetic, environmental or stochastic factors. Although the most severe presentation is presumed to be monodactyly, the mildest form may only manifest subtle digital defects or split nails. The latter observation was noted by Alan Emery in an article entitled: A problem for genetic counseling—split hand deformity [Emery, 1977]. The published family included an individual who had short thumbs as her only digital manifestation yet had a severely affected child with SHFM. There were other “affected” individuals who also had only mild digital findings. The high degree of variable expressivity made genetic counseling challenging. In his summary, Emery proposed that anyone in the family with any digital findings should be assumed to be heterozygous and at risk for having a severely affected child.
A molecular-pathogenetic strategy to classify genetic disorders of the skeleton has been proposed [Superti-Furga et al., 2001]. However, as the causative factor(s) for many SHFM patients remain elusive, this approach is not yet feasible, and is complicated by the fact that at least five loci have been mapped for the isolated form of SHFM. The five types are referred to as SHFM1-5, and represent yet another type of classification of the malformation (a genetic classification). SHFM1D represents individuals mapped to SHFM1 who also possess sensorineural hearing loss. Genotypic–phenotypic correlations at the level of the chromosomal locus have not been previously performed for SHFM and will be the primary focus of this paper, along with discussion of the relevant animal models.
METHODS
The methods utilized to ascertain patients for the genotypic–phenotypic correlation studies are described elsewhere [Elliott et al., 2005a]. Briefly, we reviewed the literature of mapped SHFM1 (chromosome 7), SHFM2 (X-linked), SHFM3 (chromosome 10), SHFM4 (chromosome 3), and SHFM5 (chromosome 2) patients. Cases were included if they had central deficiency, a large gap between digits 1 and 2 or longitudinal clefting. Relevant chromosome region searches were also performed in order to identify potential cases. Since SHFM1 (7q21) is associated with normal chromosome constitutions, balanced and complex rearrangements, deletions as well as duplications, the chromosomal region (“7q”) was searched in general. Analysis was performed on cases in which there was adequate clinical and radiographic information. In total, 48 SHFM1 cases were included. Patients from the SHFM2 (X-linked kindred) were not included in the analysis due to limited clinical and radiographic information. SHFM3 cases (10q24) identified through genetic mapping were included, providing there was adequate accompanying clinical information. In total, forty mapped SHFM3 cases (with normal chromosomes) were included. Due to the genomic rearrangement consisting of a disrupted extra copy of DACTYLIN and duplication of other genes known to be important in limb development at 10q24 [de Mollerat et al., 2003b], we reviewed the literature for patients who were trisomic for the 10q24 region. Reported patients with trisomy of this chromosomal region were reviewed and cases were included if they met the inclusion criteria. In total, seven patients who were trisomic for the 10q24 region met the inclusion criteria and were included in our analysis. With respect to the analysis, both mapped and trisomic 10q24 patients were treated as one group and are referred to as “SHFM3 patients” in the following text and accompanying figures. SHFM4 (P63) cases were identified by searching for SHFM4, P63, as well as the various syndromes associated with mutations in this gene. In total, 45 SHFM4 patients with adequate information were included, all of whom had mutations in P63. The identification of the SHFM5 locus and its association with a microdeletion at chromosome 2q31 [Goodman et al., 2002] prompted us to review the literature for cases with similar chromosomal deletions. We included patients if they were deleted for this region and met our inclusion criteria. In total, 20 SHFM5 cases were finally analyzed. A comprehensive list of the patients included in the analyses has been published elsewhere [Elliott et al., 2005].
For each case identified, numerous clinical variables (e.g., particular craniofacial findings, ectodermal involvement, mental retardation, etc.) in addition to limb specific variables were coded as either binary (present, absent) or multistate to reflect degree of severity. Descriptive epidemiology and Chi-square analysis were performed as discussed below. Subsequently, discriminant function analysis using SPSS for Windows was performed to identify the specific variables (associations) that best differentiated the genetic loci.
RESULTS
With respect to karyotype, SHFMI patients showed a variety of findings (approximately 21% had apparently normal chromosomes, 46% had an apparently “balanced” karyotype and 33% had an unbalanced karyotype). Most SHFM3 patients (85%) had normal chromosomes, while the remaining 15% had trisomy of 10q24. All SHFM4 patients had presumably normal chromosomes, while all SHFM5 patients were deleted for 2q31.
SHFM1 showed a nonsignificant excess of males (33M:15F) (P = 0.0614). The sex ratios were more normal in the other groups: SHFM3, 21M:26F; SHFM4, 22M:21F; SHFM5, 8M:11F. In addition, there was one case with ambiguous genitalia, a SHFM5 infant with a 46,XY chromosome constitution, a vaginal opening, hypoplasia of clitoris and labia minora and no gonads palpable in the groin [Slavotinek et al., 1999] and two patients with EEC syndrome and P63 mutations (SHFM4) in whom the gender was not reported [Kosaki et al., 2001; Hamada et al., 2002].
As anticipated, mental retardation varied significantly between the loci (P = 4.04 × 10−7) and was most common at those loci where karyotypic anomalies were found. It was documented in 59% of SHFM5 patients and was usually severe. This is probably an underestimate as other patients had either died or were too young to be adequately assessed. Thirty-three percent of SHFM1 patients had mental retardation that varied from mild (8%) or moderate (15%) to severe (10%). Impairment was also seen in approximately 15% of SHFM3 patients and was usually severe. In contrast, only 4% of SHFM4 patients had mental retardation.
With respect to other phenotypic findings, we will present data on other limb findings, ectodermal involvement, craniofacial findings including clefting, hearing loss, congenital heart defects, and seizures as these proved to be important locus discriminating variables.
Camptodactyly of the fingers varied significantly (P < 0.00001) between the groups and was most common at the SHFM5 locus (80% of patients). It was also seen in 28% of SHFM3 patients. It was less frequent in SHFM1 (8%) and SHFM4 (4%). Camptodactyly of the toes also showed significant variation, but was not as striking (P = 0.018). Preaxial involvement including polydactyly, triphalangeal thumb, and absent radial ray was another limb specific variable that differed significantly between the groups and has been the subject of a previous report [Elliott et al., 2005].
Since SHFM can be associated with ectodermal findings as in EEC syndrome, hair (sparse hair or alopecia), skin (freckling), nails (dysplastic, dystrophic, underdeveloped or absent), teeth (abnormally shaped, oligodontia) and lacrimal involvement were analyzed carefully (Fig. 1). As anticipated, SHFM4 showed the greatest involvement with all components of the ectoderm involved. SHFM3 patients showed only nail and/or occasional dental involvement. The lack of extensive ectodermal findings at this locus suggests this locus is more “limb specific,” particularly in the chromosomally normal patients. These clinical findings are supported by the Dac mouse, the mouse model for SHFM3, as this mouse does not show any ectodermal findings, and the phenotype is very limb specific. As the nail findings in the SHFM3 patients involved ridged or dystrophic nails rather than hypoplastic or absent nails, and their hair and lacrimal ducts were normal, the precise nail phenotype appears to distinguish “ectodermal” from “non-ectodermal” loci. Freckling of the skin and lacrimal duct involvement were only observed in SHFM4 patients suggesting that this is a P63 (SHFM4)-specific phenotype. Chi-square analysis of the ectodermal variables showed that lacrimal involvement manifests a statistically significant distribution among the loci (P < 0.00001), as did freckling (P = 0.0003), sparse hair (P = 0.003), and dysplastic nails (P = 0.0111).
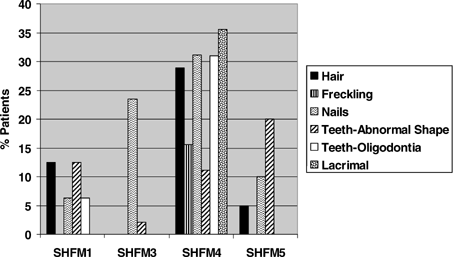
Ectodermal findings in mapped SHFM patients.
Patterns also emerged with respect to craniofacial variables (Fig. 2). Dysplastic (poorly formed or small) ears were most common in SHFM5 (40%) and SHFM1 (35%), but were rarer in SHFM3 (4%) and not reported in SHFM4 (P = 2.12 × 10−7). Importantly, ear findings in SHFM1 patients were not restricted to those with unbalanced chromosome findings [Hasegawa et al., 1991; Sharland et al., 1991; Genuardi et al., 1993; Scherer et al., 1994; Ignatius et al., 1996; Tackels-Horne et al., 2001]. Low set ears also showed significant locus variation (P < 0.00001) and were most common in SHFM5. They were not found in SHFM4. Micrognathia showed a similar distribution (P = 4.79 × 10−8). A different pattern was observed with orofacial clefts (Fig. 3). Cleft lip occurred at all loci except SHFM3, but, as expected, was most commonly seen in those with P63 mutations (P < 0.00001). Cleft palate was coded as a multistate variable ranging from mild involvement such as high arched palate to clefts of the hard palate. It also varied significantly between the loci (P = 0.0035).
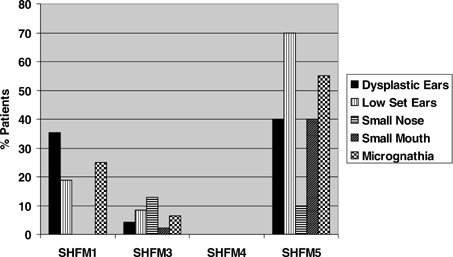
Craniofacial findings in mapped SHFM patients.
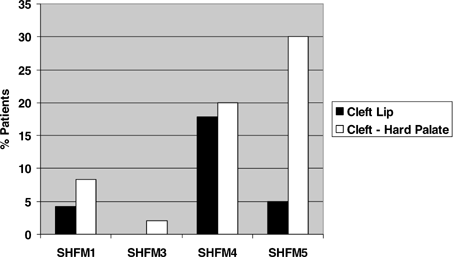
Orofacial clefting in mapped SHFM patients.
Deafness was most commonly associated with SHFM1 with a total of 35% of patients affected. In all but one of these, the hearing loss was sensorineural (70%) or mixed (conductive/sensorineural) (24%). Three of the four deaf SHFM4 patients had conductive deafness. Hearing loss was reported in only one SHFM3 case and not at all in SHFM5, thus this was a significant locus discriminator (P < 0.0001).
Seizures also varied between the groups (P < 0.001) and were most commonly found in SHFM5 with 40% affected; however, given the number of early deaths of these patients, this may be an underestimate. One SHFM1 and one SHFM3 case had seizures. Seizures were not reported in SHFM4 patients.
Congenital heart defects were found in 13% of SHFM1 patients, 6% of SHFM3 patients and in half of the patients mapped to SHFM5, but were not reported in SHFM4 patients (P = 1.09 × 10−7). All of the affected SHFM3 patients were trisomic for the 10q24 locus.
In our discriminant function analysis, an approach was utilized in which the model was constructed in a step-by-step fashion. At each step, all variables were reviewed and evaluated to identify which one had the most discriminating power between the groups. For each step, the variable that minimized the overall Wilks' Lambda (variance not accounted for) was entered. The multivariate significance at each step was P < 0.0001. The 12 major distinguishing variables were, in order of discriminating power: lacrimal involvement, camptodactyly of the upper limbs, preaxial involvement of the upper limbs, low set ears, deafness, small nose, small mouth, freckling of the skin, cleft lip, seizures, palatal involvement and a measure comparing the severity of upper and lower limb involvement (logn upper/lower) [Elliott et al., 2005] (Table I).
| Autosomal locus | SHFM1 (7q21) | SHFM3 (10q24) | SHFM4 (P63) | SHFM5 (2q31) |
|---|---|---|---|---|
| Lacrimal involvement | Absent | Absent | ++ | Absent |
| Camptodactyly: fingers | − | + | − | +++ |
| Preaxial hand involvement | − | ++ | ++ | − |
| Low set ears | + | +/− | Absent | +++ |
| Deafness | ++ | − | +/− | Absent |
| Small nose | Absent | +/− | Absent | +/− |
| Small mouth | Absent | − | Absent | ++ |
| Skin freckling | Absent | Absent | + | Absent |
| Cleft lip | − | Absent | + | +/− |
| Seizures | − | − | Absent | ++ |
| Palatal involvement (mild–severe) | ++ | +/− | + | ++ |
| Severity: hands versus feet | Feet >> Hands | Feet > Hands | Hand and foot involvement similar | Feet >>> Hands |
- +++, >67%; ++, >33%; +, >15%; +/−, 5%–15%; −, <5%.
DISCUSSION
Various authors have indicated that there is an excess of affected males with SHFM [Birch-Jensen, 1949; Froster and Baird, 1992; Froster and Baird, 1993; Czeizel et al., 1994]. However, these studies are of an epidemiologic nature and do not take specific genetic loci into consideration. In studying mapped cases, we are only considering a subset of all SHFM patients. SHFM1 did show an excess of affected males; however, this trend did not apply to the other autosomal loci. It is possible that studies documenting an excess of males included more SHFM1 or SHFM2 patients than those from the other loci. It is also possible that other loci (not yet mapped) are responsible for the more blatant gender differences observed. The mouse model of SHFM1 did not report a gender discrepancy [Merlo et al., 2002; Robledo et al., 2002]. In the mouse model of SHFM3, males and females were equally affected [Chai, 1981].
A higher frequency of affected males at an autosomal locus would suggest that males have an increased susceptibility to the mutant gene being penetrant. Potential factors explaining gender differences include difference in timing and/or susceptibility to the gene's pathogenetic effects. Apart from genetic or epigenetic explanations, there may also be a bias of ascertainment in the literature, with males more likely to be referred for clinical investigation. Potential genetic factors include X-linked modifying loci, or a mixed model for susceptibility, such as that proposed for fibular agenesis with SHFM [Evans et al., 2002].
An excess of affected males has also been reported in other limb malformations [Hay, 1971]. Differences in gender in individuals affected with SHFM could be due to differences in developmental timing. A study of prenatal hand development demonstrated that males are advanced compared to females, particularly in younger embryos (15–30 mm) [Garn et al., 1974]. There is male advancement in both the proximal hand region including the round bones of the wrist and in the distal hand region: the metacarpals and phalanges. The male embryo is advanced in the development of the hand skeleton to the time of appearance of calcified bone tissue. Since it is true separately for the proximal hand alone (including the carpals) and for the distal hand alone (metacarpals and phalanges), the authors proposed that this advanced development applies to the hand as a whole. The implications of these findings suggest that if insults are “stage-specific” one would expect critical timing to be different for male and female embryos. If insults are “time specific,” one would expect the sexes to differ considerably in the stage of embryonic development attained. Differences in congenital malformations between males and females may thus be attributable to the time lag in female fetuses with respect to limb development.
As expected, the distribution of mental retardation among SHFM patients is closely related to karyotypic abnormalities. All SHFM3 patients with mental retardation were trisomic for 10q24. All SHFM5 patients in our analysis had deletions. Only two SHFM1 patients with apparently balanced chromosome constitutions had mental retardation [Scherer et al., 1994; Ignatius et al., 1996]. One may assume that the mental retardation is due to the loss of chromosomal material—including the limb-specific genes and others potentially involved in brain development. Candidate genes for SHFM1 include DLX5 and DLX6, while candidate genes for SHFM5 include DLX1 and DLX2, suggesting common mechanisms/genetic players may be involved. Dlx genes are expressed in the developing brain. Therefore, the mental retardation seen in SHFM1 and SHFM5 patients may be explained by disruptions in the expression of the corresponding DLX genes. Dlx1 and Dlx2 are expressed in cells of the subcortical telencephalon that migrate across the pallial–subpallial limit and enter the mantle and subventricular zone (SVZ) of the cerebral cortex in embryonic day 12.5 mice. Later, Dlx1 and Dlx2 are also expressed in the interneurons of the olfactory bulb. The Dlx5 and Dlx6 genes are expressed in the developing forebrain as reviewed by Merlo et al. 2000.
With respect to limb specific variables, we have previously reported that preaxial involvement of the upper limbs was a significant limb discriminating variable. This multistate variable included mild findings such as a proximally placed thumb to triphalangeal thumb, preaxial polydactyly to absence of the preaxial rays [Elliott et al., 2005]. Preaxial involvement of the upper limbs was most common at the SHFM3 locus. The combination of findings such as triphalangeal thumb in association with cleft foot may be a phenotypic clue that the patient maps to the SHFM3 locus.
Camptodactyly of the upper and lower limbs both showed significant distribution among the four loci studied, however, it was much more striking in the upper limbs. The SHFM5 patients had a uniform camptodactyly involving most of the digits of the hands, whereas the affected SHFM3 patients tended to show involvement of one to a few digits. The index finger was most commonly involved in this patient group. It should also be noted that some of these patients had ungual involvement of this digit.
It is not surprising that ectodermal involvement was greatest at the SHFM4 locus. Although P63 testing has its limitations with isolated SHFM patients, other studies have shown that up to 93% of EEC patients have such mutations [Celli et al., 1999; Ianakiev et al., 2000; van Bokhoven et al., 2001; de Mollerat et al., 2003a]. EEC3, due to mutations in P63, is not the only EEC locus. Some families with autosomal dominant EEC1 have been linked to 7q11.2–q21.3 [Akita et al., 1993; Nunes et al., 1994; McElveen et al., 1995] and these patients are included in our SHFM1 cohort. However, although both SHFM1 and SHFM4 are considered to be EEC loci, it is important to note that there were no patients with lacrimal involvement who mapped to SHFM1, suggesting that lacrimal involvement may differentiate EEC patients who have P63 mutations from those who map to chromosome 7 or other loci. p63 null mice have no normal epidermal structures in the skin and there is complete lack of hair follicles at birth [Mills et al., 1999]. Further study of the epidermis in these mice has shown that the epidermis undergoes an unusual process of non-regenerative differentiation, resulting in the absence of all squamous epithelia and their derivatives including mammary, lacrimal, and salivary glands. P63 is therefore likely responsible for maintaining the progenitor cell populations that are required to sustain epithelial developmental and morphogenesis [Yang et al., 1999]. Although Dlx5/6 homozygous null mice (the mouse model for SHFM1) show limb defects and craniofacial changes, involvement of the lacrimal system has not been reported [Merlo et al., 2002; Robledo et al., 2002].
The freckling seen in a number of SHFM4 patients is likely due to the fact that the kindred originally described in 1993 with ADULT syndrome (acro-dermato-ungual-lacrimal-tooth syndrome) was included [Propping and Zerres, 1993]. Freckling of the skin is a component of this “EEC-like” disorder, which was subsequently found to be due to a gain of function P63 mutation [Duijf et al., 2002]. Skin findings in EEC syndrome generally consist of fair, thin skin with mild hyperkeratosis [Jones, 1988]. As with lacrimal involvement, skin freckling is not associated with the EEC1 locus at 7q, but rather with the EEC3 locus at 3q.
The association of SHFM with oral facial clefting is well established, primarily due to the association of ectrodactyly with clefting in EEC syndrome. Since cleft palate is a relatively common birth defect, it could occur occasionally with SHFM and may not be pathogenetically related. The single patient with cleft palate in a SHFM3 pedigree [Roscioli et al., 2004] may represent such a case. The lack of oral facial clefting in the Dac mouse supports what was seen (or rather not seen) in SHFM3 patients.
However, as clefting is seen in the Dlx5/6 homozygous null mouse, it is not surprising to see it in SHFM1 patients. As with the limb findings, the differences with respect to sensitivity to dosage of DLX5/6 between mouse and humans suggest that humans are much more sensitive to disruptions in DLX5 and DLX6 as the mouse heterozygotes are unaffected. Furthermore, the EEC1 patients mapped to 7q and included in the SHFM1 group show oro-facial clefting. Similarly, the high frequency of clefting at the SHFM4 locus is not unanticipated. Clefting was also reported in SHFM5 patients. The palatal processes of the maxillary bone are absent in the Dlx1/Dlx2 mutant mouse, thus cleft palate is seen in 100% of Dlx1/Dlx2 double mutant mice and in approximately 80% of Dlx2 mice and 10% of Dlx1 mutants [Qiu et al., 1997].
When considering certain craniofacial characteristics, we again see similar findings with the SHFM1 and SHFM5 loci, where DLX genes are candidates. As the expression pattern of Dlx genes includes the craniofacial primordia [Merlo et al., 2000], it is not surprising that, a craniofacial phenotype can accompany SHFM1. Dlx5/6 homozygous null mice have small eyes, unrecognizable ear structures and clefting and dysmorphogenesis of nasal, maxillary, and mandibular structures [Robledo et al., 2002]. Therefore, the SHFM1 mouse model supports the craniofacial phenotype seen in some SHFM1 patients. Interestingly, mice deleted for Dlx5 only do not show such extreme craniofacial defects [Acampora et al., 1999; Depew et al., 1999]. Thus, the differences seen in SHFM1 patients may be a reflection of the extent of the involvement of the two candidate genes. Dlx1 and Dlx2 are expressed in the proximal and distal first and second branchial arches and a variety of craniofacial bones are disrupted in Dlx1/Dlx2 homozygous mutant mice [Qiu et al., 1997]. The craniofacial findings seen in SHFM5 patients may result from related pathogenetic mechanisms.
It is proposed that Dlx genes regulate intercellular signaling across the interface between neural and non-neural ectoderm that is critical for the induction and pattern of adjacent cell fates. The lateral border of the neural plate provides patterning cues to mesodermal structures including the somites and heart [Woda et al., 2003]. Therefore, although Dlx genes may not be directly expressed in the heart, their involvement in influencing patterning of the heart may potentially explain the heart defects seen in SHFM1 and SHFM5 patients. Conversely, the Dac mouse does not exhibit craniofacial or cardiac defects and therefore supports the lack of abnormal findings in most SHFM3 patients [Chai, 1981].
Although not included in our analysis, SHFM2 patients are reported to lack ectodermal involvement, craniofacial dysmorphism (including clefting), mental retardation and other malformations suggesting this locus is very “limb specific” [Faiyaz ul Haque et al., 1993; Faiyaz-Ul-Haque et al., 2005].
It is not surprising that deafness was most common at the SHFM1 locus, due to the presence of the SHFM1D families. For SHFM1D families, the deafness is primarily sensorineural, yet some individuals show mixed hearing loss. Although deafness is associated with the EEC syndrome (present in approximately 14% of patients), the deafness in this disorder is primarily conductive [Roelfsema and Cobben, 1996]. The inner ear capsule and middle ear cartilages are fused and severely dysmorphic in Dlx5/6 null mice [Robledo et al., 2002]. Although Dlx1 and Dlx2 are expressed in the proximal and distal first and second arches, only the proximal regions are affected [Qiu et al., 1997]. Deafness, due to its well-recognized association with SHFM1 appears to be an important locus differentiating variable. Chromosomal alterations may not be necessary in SHFM1 patients for this abnormality to be manifested. This close association of deafness with SHFM1 has already resulted in the presumption that a patient with SHFM and deafness is, in fact, an SHFM1 patient [Debeer, 2004]. However, no studies were performed to confirm that this patient mapped to chromosome 7.
Split hand foot and other limb deficiencies are often so striking that it is not surprising that reports of individuals with such defects tend to focus on the limbs and may overlook accompanying craniofacial defects, which may be subtle, or indeed other phenotypic findings. Recently, we reported that a facial phenotype suggestive of Kabuki syndrome was found in a patient with Cenani–Lenz syndactyly (CLS) [Elliott et al., 2004]. Another report of CLS patients indicated these patients had mild craniofacial changes [Temtamy et al., 2003]. It is therefore important to document all associated craniofacial and other clinical findings in patients with limb findings, and in SHFM in particular, to determine if there are phenotypic findings that may allow a specific locus to be implicated.
In summary, SHFM is a complicated limb phenotype that shows extreme variability in phenotype and genetic heterogeneity. It can be isolated or associated with a variety of clinical and craniofacial findings. The limb findings of preaxial involvement and camptodactyly of the upper limbs were found to be important locus discriminators. Craniofacial findings such lacrimal involvement, palatal involvement, low set ears, small nose and mouth were also found to be important in both descriptive epidemiologic studies and subsequent discriminant function analysis. Hearing loss was another significant clinical variable. Comparison of relevant animal models supports the clinical variables which were present (and lacking) in corresponding SHFM patients. Genotypic–phenotypic correlations were thus established at the chromosomal level that we hope will assist mapping efforts and ultimately benefit patients in the clinical setting. Our next step will be to attempt to apply the algorithms generated by our analyses to previously unmapped cases to determine their sensitivity and specificity.




