Cloacal exstrophy in an infant with 9q34.1-qter deletion resulting from a de novo unbalanced translocation between chromosome 9q and Yq
Abstract
Cloacal exstrophy is a rare malformation, belonging to a spectrum of birth defects, which, in order of severity, includes phallic separation with epispadias, pubic diastasis, bladder exstrophy, and cloacal exstrophy. This malformation overlaps the OEIS complex (O = omphalocele, E = bladder exstrophy, I = imperforate anus, S = spinal defects). The etiology of cloacal exstrophy is unknown to date. It may result from either a single defect of early blastogenesis or a defect of mesodermal migration during the primitive streak period. We report an infant with cloacal exstrophy, exomphalos, right kidney agenesis, ambiguous external genitalia, and axial hypotonia. The karyotype showed a de novo unbalanced translocation between the long arm of chromosome 9 and the long arm of chromosome Y resulting in a 9q34.1-qter deletion. Reviewing the literature, we did not find any observation of cloacal exstrophy associated with a structural chromosomal abnormality. The steroidogenic factor 1 (SF1) gene, included in the deleted region, was a good candidate gene but no pathogenic mutation was found by direct sequencing. We hypothesize that another gene, expressed early in embryogenesis and responsible for cloacal exstrophy, is present in the 9q34.1-qter region. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Cloacal exstrophy (CE) is a rare malformation, which occurs in 1:200,000 to 1:250,000 live births [Carey et al., 1978; Martinez-Frias et al., 2001]. Most cases of CE are sporadic and isolated and their cause is unknown. However, familial cases of CE and monozygotic twins with CE [Lee et al., 1999; Keppler-Noreuil, 2001] suggest an underlying genetic mechanism in some situations. CE is sometimes associated with other malformations including the central nervous and skeletal systems [Martinez-Frias et al., 2001]. In this study, we report an infant with CE and a de novo unbalanced translocation between the long arm of chromosome 9 and the long arm of the chromosome Y resulting in a 9q34.1-qter deletion.
CLINICAL REPORT
The proband was born from nonconsanguineous parents. The mother was 25 years old and the father was 31 years old at time of pregnancy. They had three healthy children. Family history was unremarkable. There was no exposure to drugs or infections during pregnancy. Prenatal ultrasound surveys were normal.
Growth parameters at birth (36 weeks of gestation) were 2,560 g for weight (10th centile), 46 cm for length (5th centile), and 31.5 cm for OFC (5th centile). Clinical examination revealed an exomphalos associated with a cloacal exstrophy, that is, two hemibladders lying on either side of exstrophic bowel. The proximal orifice of the bowel was located medially beneath the exomphalos and allowed passage of stools (Fig. 1). He had an imperforate anus. Bifid penis was observed on widely separated pubic bones. No facial dysmorphism was noted. There was no spinal defect. Abdominorenal ultrasound showed right kidney and absent testis. Adrenal glands were normal. MRI scan of the brain, skeletal survey, thoracic X-rays, and cardiac examination were normal. CE was diagnosed.
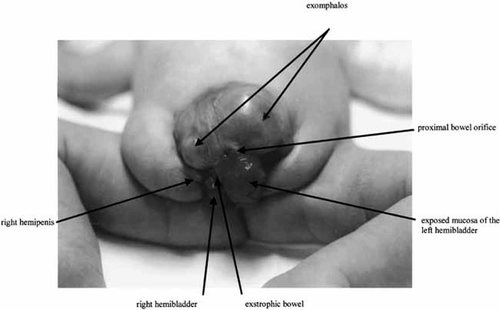
Picture of the proband showing an exomphalos associated with a cloacal exstrophy, that is, two hemibladders lying on either side of exstrophic bowel. The proximal orifice of the bowel was located medially beneath the exomphalos. He had no anus. Bifid penis was observed on widely separated pubic bones. Distal bowel orifice and ureteral meatus are not obvious on this picture.
Care in neonatal unit was initially required because of the need of enteral feeding during the first 3 weeks of life and therefore for the persistence of severe pain necessitating morphine therapy. Because of the severity of CE, curative surgery was not indicated. A colostomy was discussed but not performed since the child presented an unexplained episode of severe sepsis leading to death at 6 months of age. His last neurological survey revealed persistent hypotonia, horizontal nystagmus, and stereotypes. The parents refused autopsy.
CYTOGENETIC AND MOLECULAR ANALYSIS
Cytogenetic analyses were performed on peripheral blood lymphocytes, using GTG, RHG, and CBG banding techniques. Karyotype showed an unbalanced translocation involving the long arm of chromosome 9 and the long arm of chromosome Y (Fig. 2A). The parents karyotypes were normal.
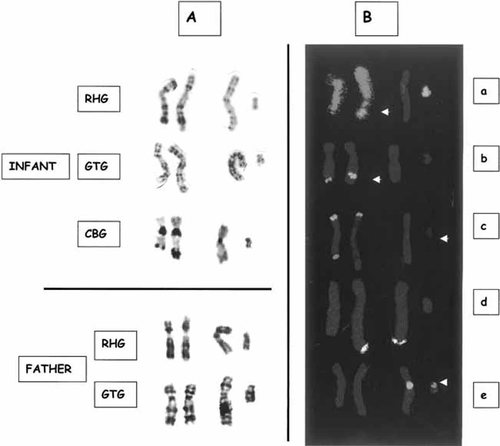
A: Partial karyotype showing the unbalanced translocation between chromosome 9 and Y (46,X,der(Y)t(Y;9)(q11.23;q34.1)del(Y)(q11.2),der(9)t(Y;9)) in the proband and normal karyotype in his father. B: Fluorescent in situ hybridization (FISH) studies in the probant. a: Chromosome painting with orange WCP9 and green WCPY showing additional material originating from chromosome Y on chromosome 9q, but the absence of material originating from chromosome 9 on chromosome Yq; (b) FISH study of brc/abl gene (LSI bcr/abl ES dual color translocation probes: bcr green, abl orange) showing one spot on both chromosomes 9q, confirming the terminal 9q deletion with a 9q34.1 breakpoint distal to the abelson gene. c/d: FISH analyses with telomeric probes of chromosome 9q (ToTelVysion probe mixture 9: 9p green, 9q orange) and Yq (ToTelVysion probe mixture 2: Xq green and orange, Yq green and orange) confirming the unbalanced translocation. e: LSI unique sequence DNA FISH probe for SRY (SRY orange, CEPX green) confirming the presence of the SRY gene. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In order to define the translocation breakpoints and the extent of the deletion, fluorescent in situ hybridization (FISH) experiments were performed on metaphase spreads using various DNA probes. Chromosome painting with WCP9 and WCPY showed additional material originating from chromosome Y on chromosome 9q, but no material originating from chromosome 9 on chromosome Yq (Fig. 2Ba). FISH analysis with telomeric probes of chromosome 9q (ToTelVysion probe mixture 9) and Yq (ToTelVysion probe mixture 2) confirmed the unbalanced translocation (Fig. 2Bc,d). FISH study of brc/abl gene (LSI bcr/abl ES dual color translocation probes) showed one spot on every chromosome 9q, confirming the terminal 9q deletion with a breakpoint located in 9q34.1 distal to the abelson gene (Fig. 2Bb).
LSI unique sequence DNA FISH probes for SRY showed the presence of the SRY gene (Fig. 2Be). FISH characterization made it possible to define the patient's karyotype as follows: 46,X,der(Y)t(Y;9)(q11.23;q34.1)del(Y)(q11.2),der(9)t(Y;9).
Analysis of the SF1 gene was carried out according to previously reported techniques by direct sequencing on genomic DNA. No pathogenic mutation was found in the coding sequence of the gene [Oba et al., 1996].
DISCUSSION
In this study, we report a patient with CE and a de novo unbalanced translocation t(Y;9)(q12;q34.1), resulting in a 9q34.1-qter deletion. To our knowledge, this is the first report of a child with CE and a structural chromosomal abnormality.
CE is reported as isolated or associated with other malformations. CE may result from several possible embryological mechanisms including a single defect of early blastogenesis at approximately 29 days of development [Opitz, 1993; Lee et al., 1999] or a defect of mesodermal migration during the primitive streak period [Pauli, 1994]. In 1978, Carey et al. proposed the acronym “OEIS complex” (O = omphalocele, E = bladder exstrophy, I = imperforate anus, S = spinal defects) for a series of cases with abnormal body wall development. Since then, some authors stated that OEIS complex and CE are similar malformations [Carey, 2001; Martinez-Frias et al., 2001; Bohring, 2002]. Moreover, some authors consider CE and bladder exstrophy (BE) as either part of a continuum [Hendren, 1998] or two distinct disorders [Cadeddu et al., 1997]. Recently, pointing out this confusion in the nosology and nomenclature of these disorders, epidemiological studies showed that CE and BE are two different expressions of a primary polytopic developmental field defect, CE representing the manifestation of an earlier hit in the development [Källén et al., 2000; Martinez-Frias et al., 2001].
In the majority of cases, CE occurs as a sporadic event of unknown origin. Rare familial cases and a higher incidence of CE among monozygotic twins than among dizygotic twins suggest a genetic origin [Lee et al., 1999; Keppler-Noreuil, 2001]. No chromosomal rearrangements have been reported in patients with CE other than a report of CE with a 47,XXX karyotype [Lin et al., 1993]. Interestingly, our patient presented with a pure terminal 9q34.1-qter deletion, resulting from a de novo translocation between chromosome 9q34.1 and the heterochromatin region of chromosome Y. A review of the literature revealed 14 observations of pure partial 9q deletion with variable breakpoints [Newton et al., 1972; Smith et al., 1973; Jenkins et al., 1976; Wisniewski et al., 1977; Turleau et al., 1978; Ying et al., 1982; Schinzel, 1988; Farrell et al., 1991; Park et al., 1991; Pfeiffer et al., 1991; Schimmenti et al., 1994; Shimkets et al., 1996; Ayyash et al., 1997; Kleyman et al., 1997]. Only three of them included 9q34.1-qter region [Schimmenti et al., 1994; Ayyash et al., 1997; Kleyman et al., 1997] and no characteristic phenotype emerged (Table I). Only facial anomalies and hypotonia were consistently found. Abdominal wall defects were not described in any other observations.
| Authors |
Schimmenti et al. [1994] |
Ayyash et al. [1997] |
Kleyman et al. [1997] |
Present report |
|---|---|---|---|---|
| Growth retardation | <5th Centile | 10th Centile | 60th Centile | 10th Centile |
| Facial dysmorphism | Microcephaly | Microcephaly | Hypertelorism | |
| Broad forehead | Receding forehead | Low-set ears | ||
| Down-slanting palpebral fissures | Hypertelorism | Micrognathia | ||
| Flat nasal bridge | Webbed of neck | |||
| Epicanthus | Down-slanting palpebral fissures | |||
| Small nose with anteverted nares | Epicanthius | |||
| Protruding tongue | ||||
| Neonatal tooth | Neonatal tooth | |||
| Low-set ears | Low-set ears | |||
| Prominent occiput | Flat occiput | |||
| CNS abnormalities | Hypotonia | Hypotonia | Hypotonia | |
| Mental retardation | Nystagmus | |||
| Nystagmus | Stereotypies | |||
| Profound hearing loss | ||||
| Spine abnormalities | Sacral dimple | |||
| Genitourinary abnormalities | Hypospadias | Ambiguous external genitalia | ||
| Abdominal wall defects | Cloacal exstrophy | |||
| Karyotype | 46,XY,del(9)(q34.3) | 46,XY,del(9)(q34.3) | 46,XY,der(9)inv(9)(q31:q34.1), del(9)(q34.1) | 46,X,der(Y)t(Y;9)(q11.23:q34.1) del(Y)(q11.2),der(9)(Y:9) |
| Origin | De novo | De novo | De novo | De novo |
- CNS, central nervous system.
SF1 is an orphan nuclear receptor regulating the transcription of an array of genes involved in reproduction, steroidogenesis, and male sexual differentiation [Morohashi, 1999; Zhao et al., 2001]. It regulates the regression of Mullerian structures and was cloned by Oba et al. [1996]. Heterozygous mutations have been described in a 46,XY patient presenting with male pseudohermaphrodism and adrenal failure in the first 2 years of life [Achermann et al., 1999], and in a female patient with isolated adrenal insufficiency [Biason-Lauber and Schoenle, 2000]. Thus, authors conclude that SF1 has a crucial role in adrenal gland formation in both sexes and in male gonadal differentiation but is probably not necessary for female gonadal development. SF1 appeared a good candidate gene in our patient but no mutation was found in the coding sequence. Thus, there may be another in the 9q34.1 region playing a role in the cause of CE.




