Sibpair studies implicate chromosome 18 in essential hypertension
Abstract
Interest in chromosome 18 in essential hypertension comes from comparative mapping of rat blood pressure quantitative trait loci (QTL), familial orthostatic hypotensive syndrome studies, and essential hypertension pedigree linkage analyses indicating that a locus or loci on human chromosome 18 may play a role in hypertension development. To further investigate involvement of chromosome 18 in human essential hypertension, the present study utilized a linkage scan approach to genotype twelve microsatellite markers spanning human chromosome 18 in 177 Australian Caucasian hypertensive (HT) sibling pairs. Linkage analysis showed significant excess allele sharing of the D18S61 marker when analyzed with SPLINK (P = 0.00012), ANALYZE (Sibpair) (P = 0.0081), and also with MAPMAKER SIBS (P = 0.0001). Similarly, the D18S59 marker also showed evidence for excess allele sharing when analyzed with SPLINK (P = 0.016), ANALYZE (Sibpair) (P = 0.0095), and with MAPMAKER SIBS (P = 0.014). The adenylate cyclase activating polypeptide 1 gene (ADCYAP1) is involved in vasodilation and has been co-localized to the D18S59 marker. Results testing a microsatellite marker in the 3′ untranslated region of ADCYAP1 in age and gender matched HT and normotensive (NT) individuals showed possible association with hypertension (P = 0.038; Monte Carlo P = 0.02), but not with obesity. The present study shows a chromosome 18 role in essential hypertension and indicates that the genomic region near the ADCYAP1 gene or perhaps the gene itself may be implicated. Further investigation is required to conclusively determine the extent to which ADCYAP1 polymorphisms are involved in essential hypertension. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Hypertension is a multifactorial disorder in which multiple genes interact with environmental factors to influence the expression of the disorder. Interest in chromosome 18 in essential hypertension comes from a previous study in which comparative mapping of rat blood pressure quantitative trait loci (QTL) was used to identify homologous regions in the human genome [Stoll et al., 2000]. A region on the q arm of chromosome 18 in humans shows similar homology to a blood pressure QTL on rat chromosome 15 [Stoll et al., 2000]. Previous studies have also shown that the 18q region is implicated in blood pressure regulation in humans. DeStefano et al. [1998] showed that 18q is linked to a rare familial disorder termed familial orthostatic hypotensive syndrome whilst Pankow et al. [2000] who examined postural changes in blood pressure in a large collection of hypertensive (HT) sibs also implicated 18q [Pankow et al., 2000]. Chromosome 18 also contains a number of potential hypertension candidate genes including the adenylate cyclase activating polypeptide 1 (ADCYAP1) and the melanocortin receptors, MC2R and MC5R, which are receptors for the glucocorticoidic corticotrophin [Human Genome Project Working Draft, 2002]. Interestingly, ADCYAP1 markedly raises glucocorticoid production [Andreis et al., 1995] and has hypotensive properties on the microcirculation [Whalen et al., 1999]. Adenylate cyclase activating polypeptide is also known to exert central nervous system effects such as neurotransmission, neuronal growth, and differentiation and peripheral effects such as increasing intracellular calcium concentration and activation of adenylate cyclase/protein kinase A and/or phospholipase C/protein kinase C [Baron et al., 2001]. Adenylate cyclase activating polypeptide in addition appears to have potent insulinotropic effects in pancreatic cells and exhibits effects on cardiac function by modulating cardiac ATP-sensitive potassium channels [Baron et al., 2001]. The gene coding for adenylate cyclase activating polypeptide is a member of a superfamily, which includes glucagon, glucagon-like peptide-1, growth hormone releasing hormone [Sherwood et al., 2000] all of which are possible hypertension candidate genes. ADCYAP1 regulates metabolism and the cardiovascular, endocrine, and immune systems, although the physiological event(s) that mediate these responses remains to be identified [Sherwood et al., 2000]. Finally a LOD score of 2.1 on chromosome 18 in the vicinity of the MC5R and ADCYAP1 genes was obtained when a genome wide scan for long-term systolic and diastolic blood pressure phenotypes was conducted in the largest families from two generations of the Framingham study [Levy et al., 2000]. Given the increasing evidence for involvement in essential hypertension, the ADCYAP1 gene may therefore be a potential candidate gene in hypertension.
To further investigate involvement of chromosome 18 in human essential hypertension, the present study utilized a linkage scan approach and, in addition, investigated the role of the ADCYAP1 gene in essential hypertension. The chromosome 18 investigation of the present study was undertaken as part of a full genome scan, recently completed [Griffiths et al., unpublished data]. Twelve microsatellite markers spanning human chromosome 18 were tested for linkage to hypertension in 177 HT sibling pairs. In addition a microsatellite variant located in the 3′ untranslated region of ADCYAP1 was tested for both linkage and association.
MATERIALS AND METHODS
Ethical clearance was sought and approved by Griffith University's Ethics Committee for Experimentation on Humans before commencing the study. Blood was collected from 239 Caucasian HT siblings (blood pressure ≥140/90 mmHg prior to anti-HT medication) through public advertisements, general practitioners, and the National Health and Medical Research Council of Australia (NHMRC) Twin Registry. Of the complete pairs collected by the twin registry, only HT dizygotic twins were selected for this study with zygosity being determined by typing at least eight highly polymorphic microsatellite markers, the HFE genotypes, and the ABO, Rh, and MNS blood group systems [Whitfield et al., 2000]. This population provided linkage information for a total of 177 sibpair comparisons from 117 nuclear families, comprised of 95 pairs, 16 trios and 6 quartet affected families and of 82 female:female, 35 male:male, and 60 female:male affected pairs. The average age and body mass index (BMI) of this tested population was 55 ± 11 years and 27.2 ± 5.2, respectively, with pretreatment blood pressures (BP) of 169.4 ± 24.0 mmHg systolic BP and 102.2 ± 10.2 mmHg diastolic BP. In addition, for the cross-sectional allelic association study, blood was collected from genetically predisposed HT and normotensive (NT) populations, as described elsewhere [Rutherford et al., 2001]. Specifically, samples were collected from 135 HTs with blood pressure ≥140/90 mmHg prior to medication and who were the offspring of two HT parents and from 101 NTs with blood pressure <140/90 mmHg and who were the offspring of two NT parents. All recruited individuals for the study gave informed consent and were adult Caucasians of British descent living in Australia, having emigrating ancestors within the last 160 years from various locations within the British Isles. Local aggregation of genetic traits, noted previously in some highly consanguineous populations in discrete regions within the United Kingdom, is therefore unlikely in these recruited populations. All participants completed a detailed questionnaire to obtain demographic parameters, to determine ancestry and to exclude those with a family history of diabetes, renal, heart, and thyroid disease. From this larger cohort of HT and NT individuals, age, sex, and ethnically matched HT and NT populations were selected. This larger cohort of individuals was also subdivided into obese and non-obese individuals depending on BMI. Obese individuals had a BMI ≥26 kg/m2 and non-obese individuals had a BMI <26 kg/m2 as previously described [Rutherford et al., 1997].
Genotyping
Genomic DNA was extracted from blood samples and markers genotyped using PCR and capillary electrophoresis using previously published methods [Rutherford et al., 2001]. Microsatellite markers spanning chromosome 18, as indicated in Table I, were analyzed. In addition, a microsatellite marker located within the 3′ untranslated region of the ADCYAP1 gene was also analyzed. Primer sequences for the microsatellite markers spanning chromosome 18 were obtained from both published reports [Gyapay et al., 1994; Dib et al., 1996] and The Genome Database [1998]. Primer sequences flanking a microsatellite marker located within the 3′UTR of the ADCYAP1 gene have also been previously described [Perez-Jurado and Francke, 1993] (ADCYAP1: chromosome 18p11 dinucleotide marker with heterozygosity of 0.8318 and allele sizes between 101 bp and 125 bp). All PCR products were genotyped using an ABI PRISM™ 310 Genetic Analyzer with Genescan Software (Applied Biosystems, Foster City, CA) and markers spanning chromosome 18 were normalized to Centre d'Etude du Polymorphisms Humain (CEPH) DNA which acted as a positive control.
| Locus | cMb | Base pairs | SPLINKa | ANALYZE (Sibpair) | MAPMAKER SIBS | |||
|---|---|---|---|---|---|---|---|---|
| LOD | P-value | LOD | P-value | LOD | P-value | |||
| D18S59 | 0.00 | 147–167 | 1.17 | 0.016 | 1.19 | 0.0095 | 1.06 | 0.014 |
| ADCYAP1c | 101–125 | 0.00 | 0.57 | 0.00 | 0.50 | 0.00 | 0.50 | |
| D18S52 | 9.26 | 114–130 | 0.09 | 0.33 | 0.00 | 0.50 | 0.09 | 0.26 |
| D18S452 | 18.70 | 123–141 | 0.00 | 0.59 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S464 | 31.17 | 296–311 | 0.00 | 0.58 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S53 | 41.24 | 148–178 | 0.00 | 0.58 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S474 | 71.32 | 118–140 | 0.00 | 0.58 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S64 | 84.80 | 311–337 | 0.23 | 0.21 | 0.03 | 0.36 | 0.21 | 0.16 |
| D18S68 | 96.48 | 266–290 | 0.00 | 0.59 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S61 | 105.03 | 205–235 | 3.22 | 0.00012 | 1.25 | 0.0081 | 2.99 | 0.0001 |
| D18S469 | 109.18 | 205–235 | 0.00 | 0.59 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S462 | 120.05 | 213–225 | 0.00 | 0.58 | 0.00 | 0.50 | 0.00 | 0.50 |
| D18S70 | 126.00 | 115–135 | 0.00 | 0.59 | 0.02 | 0.37 | 0.00 | 0.50 |
- Markers in bold indicate nominal significance (P < 0.05) with evidence for excess allele sharing. The D18S61 marker however, suggests linkage at the genome wide level (P < 0.0007) [Lander and Kruglyak, 1995].
- a Conservative weighted SPLINK values reported.
- b Sex averaged genetic distances obtained from The Centre for Medical Genetics (http://research.marshfieldclinic.org/genetics/).
- c Position of ADCYAP1 is highlighted between D18S59 and D18S476 (Fig. 1).
Statistical Analysis
Genotypes for the affected sibpairs were assessed and analyzed for linkage using identity by descent (IBD) nonparametric methods. The extent of allele sharing among affected sibpairs was determined using SPLINK (version 1.04) [Holmans, 1993; Holmans and Clayton, 1995], ANALYZE (Sibpair) (version 2.1) [Terwilliger, 1996], and MAPMAKER SIBS (version 2.0) [Kruglyak and Lander, 1995] statistical packages. In the present study, three linkage programs calculating IBD allele sharing and freely available from http://linkage.rockefeller.edu/soft/list.html were used because at present, it was unknown which is the most appropriate and powerful method of analyzing data in complex disorders using the affected sibpair approach. Prior to allele sharing calculation, estimates of allele frequencies were internally calculated from the HT sibship data using GCONVERT (version 0.96.8) [Duffy, 1998]. These allele frequency estimates were then used for the ANALYZE (Sibpair) and MAPMAKER SIBS programs. The SPLINK program automatically calculated maximum-likelihood estimates of the allele frequencies internally from the HT sibship data.
Maximization of the likelihood ratio for SPLINK analysis was restricted to the possible triangle restriction: z[1] < 0.5 and z[0] < 0.5 × z[1], where z[1] and z[0], are the sharing of 1 and zero alleles IBD, respectively [Holmans, 1993; Holmans and Clayton, 1995]. The conservative weighted test statistic of SPLINK was used for discussion purposes. SPLINK weighted individual sibpair comparisons for larger sets of affected sibs by a factor of 2/A, where A is the number of affected sibs in a nuclear pedigree [Holmans, 1993; Holmans and Clayton, 1995]. The extent of allele sharing calculated by MAPMAKER SIBS also uses the possible triangle restriction as previously described for SPLINK. Allele frequency estimates for MAPMAKER SIBS and ANALYZE (Sibpair) were externally calculated using GCONVERT and compiled into a data file for input into these statistical packages. No data file for SPLINK was required as allele frequency estimates were calculated internally using the imputed pedigree file. Analysis by MAPMAKER SIBS was in single point mode, analysis of all affected sibpairs (pair option 3) was weighted and the assumption of dominance variance was selected due to the application of Holman's triangle. A weighted statistic was also reported by the ANALYZE (Sibpair) program for n sibs (in a given pedigree) resulting in n-1 sibpair comparisons. No parental genotype information was present therefore, the more powerful all affected sibs (A/A) partition was calculated and reported for the likelihood of each possible genotype for the untyped parents [Terwilliger, 1996]. Calculation of alleles shared IBD in a sibpair over all the possible parental combinations weighted by their conditional probabilities was provided [Terwilliger, 1996]. The overall means test comparing the number of alleles shared IBD among all sibpairs with the number of alleles not shared IBD among all sibpairs is reported.
Multipoint linkage analysis was performed using MAPMAKER SIBS (version 2.0) encompassing a scan of all selected ABI Prism chromosome 18 panel markers at 0.0 cM beyond the ends of the map and in increments of two steps per map interval. The pairs used command was activated to accommodate sibships with >2 affected HT sibs. As with single point analysis, MAPMAKER SIBS multipoint analysis was performed under the assumption of dominance variance. Allele frequency estimates calculated by GCONVERT were compiled for each chromosome marker and then submitted into a chromosome 18 datafile.
Allelic association results for the ADCYAP1 marker were analyzed using a Pearson chi-square test and Clump analyses with Monte Carlo simulations [Sham and Curtis, 1995]. Clump analysis overcomes problems with low cell numbers associated with highly polymorphic markers with multiple alleles. This program performs Monte Carlo simulations to calculate four chi-squared P value tables based on the probability that the observed data generated between the case and control populations have occurred by chance. We focussed only on the T1 statistical tests from CLUMP, which is calculated from the observed chi-squared table obtained directly from the case and control alleles.
RESULTS
Chromosome 18 was scanned by genotyping twelve microsatellite markers in HT sibpairs. Linkage analyses of chromosome 18 genotypic results showed excess allele sharing with one marker, D18S59, when analyzed with SPLINK giving a P value of 0.016 (LOD = 1.2) and a P value of 0.0095 (LOD = 1.2) with ANALYZE (Sibpair) (Table I) as did MAPMAKER SIBS with a LOD score of 1.06 (P value of 0.014). The D18S59 marker is positioned close to the telomere and is physically mapped to the chromosome 18p11 cytogenetic location [The Genome Database, 1998; NCBI's Genemap, 1999; Maglott et al., 2000].
Another chromosome 18 marker, the D18S61, also showed significant excess allele sharing not only with SPLINK (P = 0.00012; LOD = 3.2) and ANALYZE (Sibpair) (P = 0.0081; LOD = 1.3), but also with MAPMAKER SIBS (P = 0.0001; LOD = 2.99) (Table I). Given that established criteria suggests a threshold of P < 0.0007 for suggestive linkage, P < 0.00002 for significant linkage, and P < 0.01 for confirmation of established linkage [Lander and Kruglyak, 1995], the MAPMAKER SIBS and SPLINK results therefore suggest that the D18S61 marker may be linked to the disease locus. Multipoint linkage analysis spanning chromosome 18 did not produce linkage peaks of interest as all generated P values were greater than the nominal significance threshold (α = 0.05).
A candidate gene association approach was also used to further investigate the hypertension susceptibility region near D18S59 on chromosome 18. A microsatellite marker located within the 3′ untranslated region of the ADCYAP1 gene was used to genotype not only HT siblings for the linkage study, but also case-control populations of HT and NT individuals for the association approach. Results from linkage analysis gave no evidence for involvement of ADCYAP1 in essential hypertension when analyzed with SPLINK (P = 0.57), ANALYZE (Sibpair) (P = 0.50), and with MAPMAKER SIBS (P = 0.50). However, analysis of allele frequencies in HT and NT populations showed significant differences for the ADCYAP1 marker (P = 0.038; Monte Carlo P = 0.02, n = 5,000) (Table II) located near the telomere and co-localized to the D18S59 marker at the chromosome 18p11 cytogenetic location.
| n | Total alleles | χ2 | P-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 112 | 114 | 116 | 118 | 120 | 122 | 124 | 126 | 128 | 130 | 132 | 134 | ||||
| Matched HT & NT | |||||||||||||||
| NT | 59 | 1 | 3 | 35 | 9 | 9 | 12 | 23 | 14 | 8 | 4 | 0 | 0 | 19.2 | 0.038 |
| HT | 59 | 0 | 4 | 63 | 6 | 8 | 4 | 12 | 10 | 6 | 4 | 0 | 1 | (19.2) | (0.02) |
| Female NT | 37 | 1 | 26 | 5 | 6 | 7 | 12 | 8 | 5 | 4 | 10.3 | 0.25 | |||
| Female HT | 37 | 4 | 40 | 3 | 6 | 4 | 8 | 6 | 2 | 1 | (10.3) | (0.28) | |||
| Male NT | 22 | 1 | 2 | 9 | 4 | 3 | 5 | 11 | 6 | 3 | 0 | 0 | 0 | 22.3 | 0.014 |
| Male HT | 22 | 0 | 0 | 23 | 3 | 2 | 0 | 4 | 4 | 4 | 3 | 0 | 1 | (22.3) | (0.008) |
| NT+HT | |||||||||||||||
| Non-obese | 90 | 0 | 5 | 55 | 22 | 12 | 12 | 26 | 19 | 16 | 7 | 5 | 1 | 16.4 | 0.13 |
| Obese | 77 | 1 | 4 | 64 | 6 | 11 | 11 | 18 | 18 | 16 | 4 | 0 | 1 | (16.4) | (0.12) |
| Female non-obese | 61 | 3 | 41 | 13 | 9 | 9 | 15 | 13 | 10 | 6 | 3 | 11.9 | 0.22 | ||
| Female obese | 44 | 3 | 41 | 3 | 6 | 6 | 7 | 11 | 10 | 1 | 0 | (11.9) | (0.22) | ||
| Male non-obese | 29 | 0 | 2 | 14 | 9 | 3 | 3 | 11 | 6 | 6 | 1 | 2 | 1 | 10.1 | 0.52 |
| Male obese | 33 | 1 | 1 | 23 | 3 | 5 | 5 | 11 | 7 | 6 | 3 | 0 | 1 | (10.1) | (0.57) |
| NT only | |||||||||||||||
| Non-obese | 68 | 0 | 4 | 30 | 20 | 9 | 11 | 21 | 16 | 13 | 6 | 5 | 1 | 17.0 | 0.11 |
| Obese | 43 | 1 | 2 | 26 | 3 | 8 | 8 | 13 | 11 | 13 | 1 | 0 | 0 | (17.0) | (0.08) |
| Female non-obese | 48 | 2 | 26 | 12 | 7 | 8 | 12 | 11 | 9 | 6 | 3 | 10.5 | 0.31 | ||
| Female obese | 23 | 1 | 16 | 2 | 4 | 3 | 4 | 7 | 9 | 0 | 0 | (10.5) | (0.31) | ||
| Male non-obese | 20 | 0 | 2 | 4 | 8 | 2 | 3 | 9 | 5 | 4 | 0 | 2 | 1 | 14.6 | 0.20 |
| Male obese | 20 | 1 | 1 | 10 | 1 | 4 | 5 | 9 | 4 | 4 | 1 | 0 | 0 | (14.6) | (0.14) |
| HT only | |||||||||||||||
| Non-obese | 22 | 1 | 25 | 2 | 3 | 1 | 5 | 3 | 3 | 1 | 0 | 0 | 2.8 | 0.97 | |
| Obese | 34 | 2 | 38 | 3 | 3 | 3 | 5 | 7 | 3 | 3 | 0 | 1 | (2.8) | (0.99) | |
| Female non-obese | 13 | 1 | 15 | 1 | 2 | 1 | 3 | 2 | 1 | 0 | 1.8 | 0.99 | |||
| Female obese | 21 | 2 | 25 | 1 | 2 | 3 | 3 | 4 | 1 | 1 | (1.8) | (0.10) | |||
| Male non-obese | 9 | 0 | 10 | 1 | 1 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 1.7 | 0.98 | |
| Male obese | 13 | 0 | 13 | 2 | 1 | 0 | 2 | 3 | 2 | 2 | 0 | 1 | (1.7) | (0.99) | |
- Association results were obtained using a Pearson Chi-Squared Test and Clump analyses with 5000 Monte Carlo simulations (in brackets).
On subdividing the age, gender, and ethnically matched HT and NT populations by gender, a significant difference in ADCYAP1 allele frequencies was obtained in males (P = 0.014; Monte Carlo P = 0.008, n = 5,000) (Table II). The ADCYAP1 marker was also analyzed for association with obesity based on subdivision by BMI. Obese individuals were classified as having a BMI ≥26 kg/m2 and non-obese as having a BMI <26 kg/m2. Analysis was undertaken in the total population with results from chi-squared analyses showing no ADCYAP1 association with obesity (Table II).
DISCUSSION
Comparative mapping of blood pressure QTL on rat chromosome 15 with that on the q arm of chromosome 18 [Stoll et al., 2000], familial orthostatic hypotensive syndrome studies [DeStefano et al., 1998], and essential hypertension pedigree linkage analyses [Pankow et al., 2000] indicate that a locus or loci on human chromosome 18 may play a role in hypertension development. Results from the present study provide evidence for the location of a hypertension susceptibility locus on chromosome 18 near the D18S61 marker and also possibly near the D18S59 marker. A previous study by Levy et al. [2000] showed a two point LOD score of 1.9 for diastolic blood pressure with the D18S481 marker located 6.94 cM from the D18S59 marker (Fig. 1) and a multipoint analysis LOD score of 2.1 peaking at the 7 cM region [Levy et al., 2000] near the D18S481 marker, see Figure 1. Hence, results from the Levy et al. [2000] study support the results of the present study showing a possible susceptibility locus near the D18S59 marker located towards the telomere of chromosome 18p.
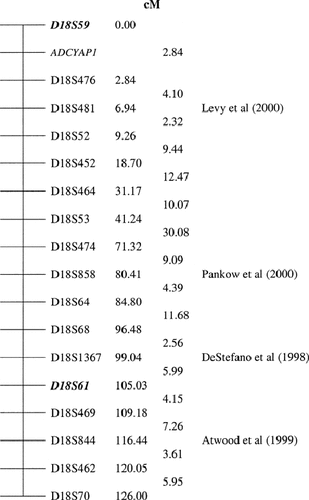
Schematic representation of Chromosome 18 and location of markers implicated in other essential hypertension investigations. Markers in bold type represent excess allele sharing at these loci (present study) with the D18S61 marker suggesting evidence for linkage at the genome wide level (P = 0.00012). The ADCYAP1 gene resides within the interval D18S59–D18S476 [NCBI's Genemap, 1999]. Sex averaged genetic distances (cM) obtained from The Centre for Medical Genetics (http://research.marshfieldclinic.org/genetics/). D18S476 was provided as the centromeric interval for ADCYAP1 and was not part of the present study's chromosome 18 scan.
The present study also provided evidence for the location of a hypertension susceptibility locus on chromosome 18q. Support for these results is also provided from chromosome 18 studies. In a Mexican American population, Atwood et al. [1999] showed suggestive linkage of hypertension to marker D18S844 located within 11 cM of the linkage to D18S61 found in our study (Fig. 1). Pankow et al. [2000] also showed suggestive evidence for linkage for the postural systolic blood pressure response with the D18S858 marker (LOD = 2.6), which is located within 25 cM of D18S61 (Fig. 1). This D18S858 marker also lies within 20 cM of the D18S1367 marker showing the highest LOD score of 3.92 for an orthostatic hypotensive syndrome study [DeStefano et al., 1998] (Fig. 1). Further evidence for a chromosome 18 role in blood pressure comes from a genome scan identifying maternal genes whose genotypes may influence the risk of developing pre-eclampsia, a condition characterized by high blood pressure during pregnancy [Moses et al., 2000]. In this study, a LOD score greater than 1.0 was shown for chromosome 18 although the exact location of the highest peak was not disclosed [Moses et al., 2000].
The present study provided some evidence for the location of at least one hypertension susceptibility locus on chromosome 18. It should be noted that these results were obtained using single point linkage analysis. Multipoint linkage analysis spanning chromosome 18 did not produce linkage peaks of interest as all generated P values were greater than the nominal significance threshold (α = 0.05). It has previously been noted that multipoint linkage analysis may falsify linkage results and could lead to exclusion of true linkage regions [Shields et al., 1991]. Also, reported LOD scores for multipoint analysis may be lower than reported two-point LOD scores in the presence of linkage [Goring and Terwilliger, 2000]. However, reported single point LOD scores must be interpreted with caution due to the potential of genotyping error. In our study, we have detected positive single point LOD scores on chromosome 18, but did not detect positive multipoint LOD scores over the same region. Although we checked for Mendelian inconsistencies using UNKNOWN from the MLINK linkage package [Terwilliger and Ott, 1994], detecting genotyping errors in our HT sibpair cohort was made more difficult due to having untyped parental information. Even though the presence of such genotyping errors could ultimately account for a reduction in the power of a study and subsequent spurious two-point linkage values [Terwilliger et al., 1990], multipoint LOD scores can be affected more greatly than two-point LOD scores in the presence of genotyping errors [Goring and Terwilliger, 2000]. Hence it is possible that linkage of chromosome 18 to hypertension exists, but in the current test population could only be detected by single point LOD scores. Implicated regions were detected by single point analysis around the D18S59 and the D18S61 markers.
The regions between D18S59 and D18S61 are separated by a very considerable distance, as much as 105 cM (Fig. 1), indicating that there is possibly more than one gene on chromosome 18 involved in blood pressure regulation. Previous studies also indicate that there may be more than one chromosome 18 gene that regulates systolic blood pressure and with much broader significance for blood pressure regulation [Atwood et al., 1999; Pankow et al., 2000]. Results from these studies therefore provide support for results of the present study showing linkage of the D18S61 marker and possible evidence for D18S59 linkage to essential hypertension.
The ADCYAP1 gene shows co-localization to the D18S59 marker [The Genome Database, 1998; NCBI's Genemap, 1999; Maglott et al., 2000]. Results from the present study testing a dinucleotide repeat located in the 3′ untranslated region of the ADCYAP1 gene showed possible ADCYAP1 association with hypertension, particularly in males. The association results of the present study therefore implicate a role for ADCYAP1 in hypertension. These results however were not supported by the linkage study, but for genes of modest effect, association studies are generally considered to be more powerful.
ADCYAP1 is located in a region implicated in the genome scan and there is evidence that it could play a role in hypertension. ADCYAP1 is known to exert vascular effects through a C-terminal 11 amino acid residue located in the ADCYAP1 gene [Ishizuka et al., 1992]. Activation of this residue ultimately affects the aorta smooth muscle cells [Ito et al., 1994] to reduce arterial pressure [Minkes et al., 1992] and vascular resistance in the coronary system [Champion et al., 1996] at low doses and biphasic changes (decreases followed by increases) in central venous pressure and cardiac output [Minkes et al., 1992] leading ultimately to essential hypertension [Ishizuka et al., 1992] at high doses. High dose of ADCYAP1 also has potent and long lasting excitatory effects on the sympathetic nervous system leading to an increase in spinal sympathetic outflow and an elevation in blood pressure [Lai et al., 1997]. Hence, the polymorphic variant located in the 3′ end of the gene may perhaps be in linkage disequilibrium with this C-terminal 11 amino acid residue and thereby affect activation of ADCYAP1. Further studies particularly of independent populations should clarify the role of ADCYAP1 in hypertension.
Acknowledgements
We thank the NHMRC Twin Registry for aid in accessing HT sibships and also volunteers from the Nambour Skin Cancer trial for providing some HT and some NT blood samples. We thank Sidney Hooker and Agnieszka Warchalowski for technical assistance and Rod Lea and Dale Nyholt for statistical analysis advice.




