Genetic testing for a BRCA1 mutation: Prophylactic surgery and screening behavior in women 2 years post testing
Abstract
Mutations in the BRCA1 gene are associated with an increased risk of breast and ovarian cancer in carrier women. An understanding of behavioral responses to BRCA1 mutation testing by mutation carriers and non-carriers is important to guide the clinical application of this new technology. This study examined the utilization of genetic testing for a BRCA1 mutation in high-risk individuals and the response of tested women with respect to interventions for early cancer detection and prevention. This study assessed the utilization of genetic testing for both men and women in a large kindred and the behavioral responses by women with respect to use of health care interventions during the 2 years following testing. Participants were offered BRCA1 mutation testing. Surveillance behaviors related to breast and ovarian cancer were assessed by computer-assisted telephone interviews at baseline (prior to genetic counseling and testing), 1–2 weeks, 4–6 months, 1 and 2 years after the provision of test results. Mutation carriers, non-carriers, and individuals of unknown mutation status were compared to determine the impact of test results. Utilization of genetic testing for both men and women are reported and, for women, mammography, breast self-exam, clinical breast exam, mastectomy, oophorectomy, transvaginal ultrasound, and CA125 screening were assessed. Of those fully informed of the opportunity for testing, 55% of the women and 52% of the men pursued genetic testing. With respect to mammography for women 40 years and older, 82% of mutation carriers obtained a mammogram in each year following testing compared to 72% of non-carrier women the first year and 67% the second year. This mammography utilization represents a significant increase over baseline for both mutation carriers and non-carriers. Younger carrier women also significantly increased their mammography utilization from baseline. Overall, 29% of the carrier women did not obtain a single mammogram by 2 years post-testing. At 2 years, 83% of the carrier women and 74% of the non-carriers reported adherence to recommendations for breast self-exam and over 80% of carrier women had obtained a clinical breast examination each year following testing. None of the carrier women had obtained a prophylactic mastectomy by 2 years after testing, although 11% were considering this procedure. Of carrier women 25 years of age and older who had at least one intact ovary at the time of testing, 46% of carriers had obtained an oophorectomy 2 years after testing, including 78% of women 40 years of age and older. The majority of carrier women (73%) had discussed their genetic test results with a medical doctor or health care provider. Our results indicate utilization of genetic testing by a majority of high-risk individuals who received information about testing. Both carriers and non-carriers increased their utilization of mammography and breast self-exam following testing. Oophorectomy was obtained by a large proportion of carrier women in contrast to mastectomy which was not utilized within the first 2 years following testing. © 2003 Wiley-Liss, Inc.
INTRODUCTION
It is estimated that approximately 5–10% of breast cancer cases in the general population are associated with heritable mutations [King et al., 1993]. Women who carry mutations in the BRCA1 or BRCA2 genes have a substantially increased risk of developing breast and ovarian cancer. The identification of the BRCA1 and BRCA2 genes has enabled the development of genetic tests for mutation analysis. Data derived from the breast cancer linkage consortium (BCLC) indicate that the breast cancer risk in female BRCA1 mutation carriers is approximately 85% by age 70 years and the ovarian cancer risk is 63% by age 70. Based on BCLC data, the cumulative risk of either cancer by this age is approximately 94% for female mutation carriers [Easton et al., 1995]. However, the BCLC data may reflect an ascertainment bias through the recruitment of families with multiple affected individuals. More recent evidence suggests that the cancer risk is lower in mutation carriers from a population unselected for family history [Easton, 1997; Devilee, 1999]. There also is evidence of an increased risk of prostate and colon cancer in BRCA1 mutation carriers [Ford et al., 1994]. Therefore, genetic testing for BRCA1 mutations is relevant to men as well, due to an increased risk of cancer and because men can transmit the mutation to their offspring.
The penetrance for breast and ovarian cancer varies between kindreds known to carry a BRCA1 mutation, perhaps due to different functional mutations within the gene, environmental influences, or other genetic factors [Narod et al., 1995]. This report concerns genetic testing for a single BRCA1 mutation in an extended kindred. The K2082 kindred was identified by linking individuals and families dispersed across the intermountain west through the use of health records and genealogical databases. K2082 is the largest kindred reported to date with an identified mutation at the BRCA1 locus [Goldgar et al., 1994]. The risk of ovarian cancer is higher and the age of onset for breast cancer is later for women in K2082 when compared with figures from the BCLC [Baty et al., 1997]. For K2082 carrier women, the risk of developing cancer by age 70 is 58% for breast cancer, 67% for ovarian cancer, and 88% for developing either cancer [Botkin et al., 1996]. The general population risk for breast cancer in the female Utah population is 6% to age 70 and 1% for ovarian cancer [Goldgar et al., 1994]. In K2082, the earliest age of onset of breast cancer was 27 years and for ovarian cancer, 34 years of age.
To better understand the clinical impact of genetic testing for cancer susceptibility, we conducted a prospective study of the behavioral and psychosocial responses to BRCA1 testing in individuals within the 2082 kindred. This study was designed to provide genetic counseling and genotype results to interested women and men within K2082 and evaluate their responses to this genetic information following the receipt of test results. This article describes utilization of genetic testing for both men and women in this kindred and the behavioral responses by women with respect to use of health care interventions during the 2 years following testing.
METHODS
The protocol and counseling content of this study have been described in detail elsewhere and were approved by the University of Utah IRB [Botkin et al., 1996; Baty et al., 1997].
Subjects
Eligible subjects were competent, adult members of K2082 from Utah and adjacent states who were able to attend two counseling sessions at the University of Utah. Approximately 20% (153/759) of the kindred members contacted by our project had been participants in earlier research at the University of Utah to identify the BRCA1 locus [Goldgar et al., 1994]. However, no individuals in the kindred had been provided genetic counseling, genotype results, or clinical genetic services prior to our project. Individuals with a history of breast or ovarian cancer were included in the recruitment. Individuals in K2082 are Caucasian of Northern European descent and the majority are members of the Church of Jesus Christ of Latter-day Saints (LDS or Mormons). The LDS church does not have doctrines relevant to the utilization of health care services.
Protocol
A letter was sent to potential subjects to inform them of the availability of individual counseling and testing for the kindred-specific BRCA1 mutation. Subjects who indicated interest were contacted and, following receipt of a signed consent form, an interviewer called the subject to conduct the baseline interview by telephone from a centralized, supervised facility.
All subjects received individual genetic counseling from a certified genetic counselor before testing and during the session in which results were provided. Genetic counseling and testing were offered without charge to the participants. Genetic counseling included detailed discussions of baseline screening behaviors and the counselors were active in health promotion for both carriers and non-carriers. Consultation from psychiatric, genetics, oncologic, and surgical services was available to all participants and their immediate family members without charge for the duration of the study. Consultations with physicians were not a required component of our protocol. Recommendations for mutation carriers and non-carriers provided at the post-test counseling session were consistent with recommendations published by the Cancer Genetic Studies Consortium [Burke et al., 1997].
The current analyses are based on 189 women who completed the baseline and post-test interviews. Data from three groups of subjects are reported. “Carriers” are individuals who all learned their positive mutation status through a genetic counseling session. “Non-carriers” are individuals who learned that they were not carriers of a BRCA1 mutation through two sources: (1) individual testing and provision of results through a genetic counseling session (n = 64 women); or (2) a negative test result of a parent or grandparent in the 2082 kindred (n = 28 women). Individuals who knew they were mutation negative (by self-report to the interviewer) by virtue of a parent's results from this study and who declined genetic counseling were not provided oral or written medical recommendations from our project. These two non-carrier groups have been combined in the following analyses since their responses to testing were similar. Subjects designated as unknown mutation status (UMS) are individuals who did not obtain testing and could not know their genetic status by virtue of a parent's test result (n = 15 women).
Data Collection
Recruitment into the study was conducted between 1995 and 1997. Interviews of subjects were conducted at entrance to the study (baseline) and at 1–2 weeks, 4–6 months, and 1 and 2 years after testing.
Measures
Demographic variables used in the analysis include age, education, annual household income, and marital status. Data included a personal history of breast or ovarian cancer or related surgery (oophorectomy, hysterectomy, or mastectomy). Women provided personal cancer history information for all other types of cancer. All subjects were asked about the number of first and second degree female relatives with a history of breast or ovarian cancer.
Health behaviors
Female respondents were asked about specific health behaviors, such as breast self-exam, clinical breast exam, mammography, transvaginal ultrasound, CA 125 testing, and surgical procedures, including mastectomy and oophorectomy.
General psychological distress
The 20-item state anxiety scale of the state trait anxiety inventory (STAI) [Spielberger et al., 1970; Spielberger, 1983]. Form X was administered at baseline and at each of the follow-up interviews. The STAI is a well-established and widely used measure of general distress.
Test-specific distress
The impact of event scale (IES) [Horowitz et al., 1979; Zilberg et al., 1982] was administered in the follow-up interviews only. The IES is a 15-item scale that measures event-related distress. In this context, the event referred to in the instructions to participants was the receipt of test results.
Statistical Analysis
Simple comparisons of behavioral responses between BRCA1 mutation carriers, non-carriers, and UMS subjects were first conducted using Pearson's chi-square statistic. This statistic was used to test the null hypothesis that carriers and non-carriers were no different with respect to the presence or absence of specific behaviors or outcomes. In general, reported chi-square statistics do not consider the UMS subjects due to the small sample size. Nonetheless, applying this statistic to our data occasionally resulted in small cell counts that may lead to incorrect P-values that assume large sample properties. To avoid this problem, we report exact P-values based on Fisher's exact test (two-tailed). Tests for differences in behavioral outcomes between groups that consider the influence of potential confounder variables were conducted by stratification (e.g., by age groups) or through statistical adjustments using multiple logistic regression. Finally, McNemar tests were used to determine whether the frequency of a behavior at baseline (e.g., mammography) changed by the 2 year post-testing interview. This test assumes that there are two measurements of a dichotomous outcome assessed on the same person and is therefore analogous to a paired t-test but for dichotomous dependent variables.
RESULTS
Utilization of Genetic Testing
Of the 759 adult men and women in the kindred who were sent an introductory letter inviting participation in the study, 500 responded and were provided full information about the project. From this group, 408 (82%) individuals completed a baseline interview, 296 (59%) attended a first genetic counseling session, and 269 (54%) requested BRCA1 mutation analysis. Therefore, 91% (269/298) of subjects who attended a counseling session chose to pursue genetic testing. Men obtained genetic testing at approximately the same rate (52%) as women (55%).
Descriptive Statistics
Table I is a description of female subjects at baseline who were informed directly or indirectly of their mutation status. The table lists women separately by younger and older age groups consistent with our analyses below. Carriers and non-carriers were comparable in terms of education (13.7 vs. 13.7 years), annual household income ($42,300 vs. $44,500), and marital status (83 married vs. 85%). Complete data were obtained on 37 female carriers and 92 female non-carriers for the following analyses.
| Variables measured at baseline | ||
|---|---|---|
| Age in years | Carriers (N = 20) | Non-carriers (N = 35) |
| Age 25–39 | ||
| Mean | 33.05 | 32.46 |
| SEa | 0.86 | 0.7 |
| Range | (27–39) | (25–39) |
| Ever diagnosed with breast or ovarian cancer | 0% | 0% |
| Had breast or ovary surgery | 15% | 8.6% |
| Ever diagnosed with other types of cancer | 0% | 0% |
| Number of 1st or 2nd degree female relatives with breast or ovarian cancer | ||
| Mean | 1.90 | 1.49 |
| SE | 0.29 | 0.21 |
| Range | (0–4) | (0–6) |
| Carriers (N = 27) | Non-carriers (N = 69) | |
| Age 40 and over | ||
| Mean | 51.15 | 53.96 |
| SE | 1.83 | 1.19 |
| Range | (40–72) | (40–78) |
| Ever diagnosed with breast or ovarian cancer | 22.22% | 2.9% |
| Had breast or ovary surgery | 77.78% | 46.4% |
| Ever diagnosed with other types of cancer | 11.11% | 1.4% |
| Number of 1st or 2nd degree female relatives with breast or ovarian cancer | ||
| Mean | 2.04 | 1.29 |
| SE | 0.16 | 0.13 |
| Range | (0–3) | (0–6) |
- a Standard error.
Health Related Behaviors and Intentions
Mammography
We assessed whether female subjects obtained mammograms during the 2 years following genetic testing. Our recommendation to subjects was that carrier women begin annual mammography at age 25 and that non-carriers begin annual screening at age 40. Table II includes data on utilization of mammography within the 12 months before the baseline interview and by 12 and 24 months following testing. Women with a history of breast cancer (n = 4) or age less than 25 (n = 19) were excluded from this analysis. Comparisons are made within groups comparing the baseline with 1 and 2 year survey results, and between groups at the 1 and 2 year survey results.
| Age group | Baseline (%) | One year (%) | Two year (%) | Test of differencesa at 1 year between carriers and non-carriers | Test of differencesa at 2 year between carriers and non-carriers | Test of differencesb between baseline and 1 year within groups | Test of differencesb between baseline and 2 year within groups |
|---|---|---|---|---|---|---|---|
| All (age 25 and over) | |||||||
| Carrier (N = 37) | 22 | 62 | 57 | P = 0.43 | P = 0.44 | P < 0.01 | P < 0.01 |
| Non-carrier (N = 92) | 30 | 53 | 49 | P < 0.01 | P < 0.01 | ||
| UMS (N = 15) | 0 | 27 | 20 | ||||
| 25–39 | |||||||
| Carrier (N = 20) | 10 | 45 | 35 | P = 0.06 | P = 0.17 | P < 0.01 | P = 0.06 |
| Non-carrier (N = 32) | 13 | 19 | 16 | P = 0.32 | P = 0.65 | ||
| UMS (N = 8) | 0 | 0 | 0 | ||||
| 40 and over | |||||||
| Carrier (N = 17) | 35 | 82 | 82 | P = 0.53 | P = 0.25 | P = 0.01 | P = 0.01 |
| Non-carrier (N = 60) | 40 | 72 | 67 | P < 0.01 | P < 0.01 | ||
| UMS (N = 7) | 0 | 57 | 43 |
- Excludes females with breast cancer history and related surgeries and females under age 25.
- a P-values are based on Fisher's two-tailed exact test.
- b McNemar test cannot be calculated because one time point measurement has no variation (no one or all subjects were adherent).
For all women age 25 and over, carriers and non-carriers increased significantly their utilization of mammography from baseline at both 1 year (P < 0.01) and 2 years (P < 0.01) post testing. Both groups of women demonstrated a modest decline in utilization in year 2 compared to year 1. Carrier women were not significantly more likely to obtain a mammogram during the first year (P = 0.43) or the second year (P = 0.44) following testing compared to non-carriers.
We further analyzed the data by younger and older age groups. For women age 40 and over, carrier women were not significantly more likely to obtain a mammogram during the first year (P = 0.54) or the second year (P = 0.25) compared to non-carriers. However, both the older carriers and non-carriers significantly increased their mammography utilization from baseline. For younger women, carriers tended to obtain a mammogram more often by 1 year (P = 0.06) and by 2 years (P = 0.17) compared to non-carriers, although these differences are not statistically significant.
Figure 1 presents adherence to mammography recommendations by year post testing for carrier women of 25 years of age and older. The results indicate that 59% of carriers had a mammogram in both of the 2 years following testing. Conversely, 29% of the carrier women had not obtained a mammogram by 2 years post-testing. Carrier women tended either to have no mammograms during the 2 year follow-up or a mammogram each year. Among women in the UMS group, none of the younger women (0/8) and 71% (5/7) of the women over 40 had obtained at least one mammogram by 2 years. Among all women, education, income, and scores at the 2 week interview for general distress (STAI), test-specific distress (IES), and perception of personal lifetime risk of breast cancer did not predict subsequent adherence to mammography recommendations based on multiple logistic regression.
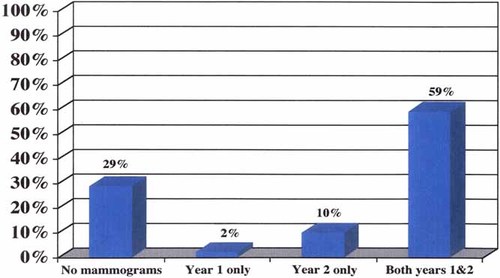
Mammography adherence by year for carrier women ≥ 25 years of age.
Breast self exam (BSE), clinical breast exam (CBE), transvaginal ultrasound, and CA-125 measurements
The American Cancer Society (but neither the National Cancer Institute or the U.S. Preventive Services Task Force) recommends that all women over 20 perform a monthly breast self-exam [American Cancer Society, 2001]. Despite the absence of data confirming the efficacy of BSE, we recommended that both carriers and non-carriers perform this examination monthly. Women were considered adherent to BSE recommendations if they reported performing BSE in 9 months or more yearly. Non-adherent women were defined as those who reported performing BSE on 8 or fewer months during the year. A CBE by a health care professional was recommended every 6 months for mutation carriers. Non-carriers were advised to obtain a CBE every 3 years from age 20 to 40 and on an annual basis over 40.
Table III includes data on utilization of BSE and CBE at baseline and by the 1 and 2 year interviews. Our results indicate that a majority of women were adherent to recommendations for BSE during the 2 years following testing and that both carrier women and non-carriers increased their utilization of BSE following testing (P < 0.01). Neither group significantly increased their utilization of CBE post testing. There was not a statistically significant difference between carriers and non-carriers for use of either BSE or CBE at 2 years. Women in the UMS group also demonstrated substantial increases in their use of BSE from baseline but not CBE.
| Baseline (%) | One year (%) | Two year (%) | Test of differencesb at 1 year between carriers and non-carriers | Test of differencesb at 2 years between carriers and non-carriers | Test of differencesb between baseline and 2 years within groups | |
|---|---|---|---|---|---|---|
| BSE | ||||||
| Carrier (N = 35) | 43 | 77 | 83 | P = 0.14 | P = 0.35 | P < 0.01 |
| Non-carrier (N = 87) | 51 | 62 | 74 | P < 0.01 | ||
| UMS (N = 14) | 21 | 57 | 79 | |||
| CBE | ||||||
| Carrier (N = 37) | 73 | 95 | 81 | P = 0.02 | P = 0.19 | P = 0.40 |
| Non-carrier (N = 91) | 73 | 77 | 69 | P = 0.61 | ||
| UMS (N = 14) | 64 | 71 | 50 | |||
| US | ||||||
| Carrier (N = 19) | 0 | 26 | 11 | P = 0.01 | P = 0.12 | c |
| Non-carrier (N = 66) | 0 | 5 | 2 | c | ||
| UMS (N = 12) | 0 | 8 | 8 | |||
| CA-125 | ||||||
| Carrier (N = 19) | 0 | 32 | 37 | P < 0.01 | P < 0.01 | c |
| Non-carrier (N = 66) | 0 | 5 | 5 | c | ||
| UMS (N = 12) | 0 | 0 | 8 |
- a P-values are based on McNemars test of agreement.
- b Ch-square P-values are based on Fisher's two-tailed exact test.
- c McNemar test cannot be calculated because one time point measurement has no variation (no one or all subjects were adherent).
Transvaginal ultrasound and CA-125 screening were recommended on an annual basis for mutation carriers beginning in their mid to late 20s. Non-carriers were not provided specific recommendations concerning early detection of ovarian cancer. Data are presented in Table III only for women with at least one intact ovary at 1 year post-testing. Our results indicate that only 26% of carrier women had obtained an ultrasound during the first year and 11% during the second year post-testing. A CA-125 measurement was obtained by 32% of carrier women the first year and 37% the second year after testing. Non-carriers and women of UMS reported low utilization rates for ultrasound and CA-125 screening.
Mastectomy
Mastectomy was discussed as an option with all carrier women. Women were informed that there were limited data on the relative efficacy of prophylactic mastectomy in the prevention of breast cancer and that complete protection from breast cancer was not assured. None of the women had obtained a mastectomy for cancer prophylaxis during the 2 year follow-up period. However, 10% (2/20) of the younger carrier women stated they were considering a mastectomy, while 12% (2/17) of the older carriers were considering this procedure at 2 years following testing.
Oophorectomy
We recommended that mutation carriers consider oophorectomy at age 35 or older, or when childbearing was complete. This recommendation was based on the conclusions of the NIH Consensus Development Panel on Ovarian Cancer [1995] and on the high incidence of ovarian cancer in K2082. Mutation carriers were informed of the limited data on the efficacy of oophorectomy for the prevention of ovarian cancer and that complete protection from ovarian cancer was not assured [Burke et al., 1997]. Of the women who proved to be carriers, 30% (11/37) had obtained an oophorectomy for a variety of indications prior to being enrolled in our study while 25% (23/92) of the non-carriers had done so. These procedures prior to baseline were done in the context of hysterectomies and, to our knowledge, were not performed specifically for cancer risk reduction.
Among all mutation carriers aged 25 and older with at least one intact ovary at baseline, 46% (12/26) reported obtaining an oophorectomy by 2 years following testing (Fig. 2). In carrier women aged 25–39, 29% (5/17) reported obtaining an oophorectomy following testing, while 78% (7/9) of the carriers 40 years and older had this procedure. For all women, income, education, family cancer history, personal cancer history, and scores on the 2-week measures for general distress and test specific distress (IES) were not predictive of a subsequent decision to obtain an oophorectomy. The small number of non-carrier women who obtained an oophorectomy following genetic testing reported that the procedure was performed for indications other than cancer prevention. None of women in the UMS group reported obtaining an oophorectomy.
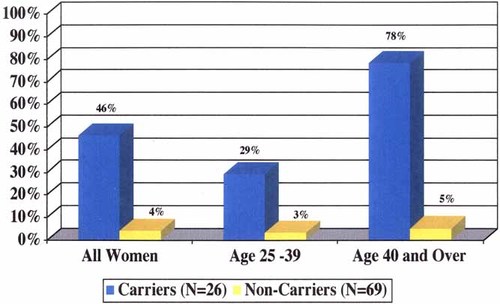
Prophylactic oophorectomy by 2 years following BRCA1 testing.
Medical consultations
Among the female carriers, 73% stated that they had discussed their genetic status with a medical doctor or health care provider following testing, while, 23% of the non-carriers reported this discussion.
DISCUSSION
Our results indicate a relatively high level of utilization of genetic testing among these at-risk men and women. The majority of both men and women who were fully informed of the opportunity for testing pursued individual genetic testing. Although the study designs differ, Lerman et al. [1996] found that 43% of high-risk adults requested results of their BRCA1 mutation status. Meijers-Heijboer et al. [2000] found that BRCA1/BRCA2 testing was requested by 48% of women and 22% of men at risk of being a mutation carrier, while Peterson et al. [2002] report that 58% of high risk women underwent BRCA1/2 testing. These studies involving actual testing demonstrate a substantially lower level of testing than was predicted from a study evaluating hypothetical interest in high-risk kindreds in which 79% of individuals indicated they “definitely” would want to have genetic testing and an additional 16% were probably interested [Struewing et al., 1995]. A study by Press et al. [2001] with women older than 40 from the general population found that greater than 70% expressed interest in genetic testing for breast cancer susceptibility. In contrast, the interest in genetic testing in kindreds studied to date with BRCA1 mutations is higher than that for genetic testing for Huntington disease, for which less than an estimated 20% of at-risk individuals have pursued genetic testing [Babul et al., 1993].
Consistent with the initial recommendations on genetic testing for cancer susceptibility [Biesecker et al., 1993; Geller et al., 1997], significant attention was paid to detailed genetic counseling and informed consent in this protocol. The pattern of responses to offers of genetic testing in our protocol suggests that the majority of individuals who decided not to pursue testing had made the decision prior to the initial counseling session. It should be emphasized that counseling and genetic testing were provided free of charge to participants in this study. Also confidentiality protections are greater within this research protocol than in an analogous clinical service because test results in clinical charts may be more readily accessible to insurance companies. Research has demonstrated that concerns about confidentiality and discrimination can be a barrier to BRCA1/2 testing [Cho et al., 1999; Peterson et al., 2002]. Both the factors of cost and enhanced confidentiality may have increased utilization of genetic testing in our study compared to what will be experienced in clinical environments involving high-risk families.
Adherence to screening recommendations is influenced by a wide variety of factors including an individual's level of anxiety and perceived risk. There is some evidence that adherence to screening recommendations is higher in women with moderate levels of anxiety and lower in women with either high or low levels of anxiety with respect to breast cancer [Lerman et al., 1990; Kash et al., 1992]. However, recent reviews suggest that adherence tends to increase with higher levels of worry [McCaul and Tulloch, 1999]. Our results demonstrate relatively high levels of adherence to recommendations for BSE, CBE, and for mammography in women 40 years and older for both carriers and non-carriers following testing. At baseline, approximately 35–40% of our female subjects 40 years and older reported having a mammogram within the past year. This compares to 47% of women in the Utah population 40 and older and approximately 56% nationally in this age group in 1996 [Centers for Disease Control and Prevention, 2000]. Among younger women, 11% of our subjects reported having a mammogram at baseline, compared to 6% in younger Utah women and 11% nationally. Therefore, our subject population was similar to women locally and nationally with respect to mammography prior to genetic testing. Involvement in the protocol substantially increased the adherence to general population recommendations for mammography and BSE for both carriers and non-carriers. Therefore, we do not find evidence in this study that women at high risk for cancer are deterred from screening, or that non-carriers are falsely reassured by genetic testing. Unfortunately, however, we found that a significant minority of carrier women (29%) had not obtained a mammogram within 2 years following testing. We did not find that general distress or test-specific distress influenced mammography adherence among carriers at 2 years post-testing.
These results contrast with results of Lerman et al. [2000] who found that female BRCA1/2 carriers were significantly more likely to obtain a mammogram compared to non-carriers (68 vs. 44%) by 1 year post testing and that the adherence rate for carriers was unchanged from baseline. In Lerman's study, cancer related distress had a positive but not statistically significant association with adherence.
Our study demonstrates a marked difference in the women's utilization of mastectomy and oophorectomy as risk-reducing measures for cancer. In a study of BRCA1/2 testing in US women, Lerman et al. [2000] found that only 3% of carrier women had obtained a prophylactic mastectomy at 1 year following testing and 13% obtained prophylactic oophorectomy. Different responses were found following BRCA1/2 testing in a Dutch population by Meijers-Heijboer et al. [2000] who found that 55% of unaffected female carriers had a prophylactic mastectomy by 2 years following testing and 60% had a prophylactic oophorectomy. Kauf et al. [2002] prospectively followed 170 BRCA1 or BRCA2 mutation carriers who were 35 years of age or older and who had not undergone bilateral oophorectomy. Of these women, 58% chose risk-reducing oophorectomy (Fig. 2).
Similar to our results, Lerman et al. [2000] document a relatively low use of CA125 testing and transvaginal ultrasound, with only 21 and 15% reporting use of these measures by 1 year. In our study, carrier women clearly preferred prophylactic surgery over early detection measures to reduce their risk from ovarian cancer. Two recent studies document substantial decreases in risk for both ovarian cancer and breast cancer for women with BRCA1 or BRCA2 mutations following oophorectomy [Kauf et al., 2002; Rebbeck et al., 2002].
The reason for the different responses between the women followed in our study and Lerman's and those studied by Meijers-Heijboer with respect to mastectomy is unclear. Certainly cultural differences are likely to be highly influential. Julian-Reynier et al. [2001] document a significant variation between cultures on attitudes toward cancer prevention strategies. Further, our data collection was largely complete before the report of Hartmann et al. [1999] that estimated a 90% reduction in risk of cancer and death from breast cancer from prophylactic mastectomy in high-risk women, however, this would have been true for the research participants in the other studies as well. The degree of risk reduction from mastectomy in women with known BRCA1 or BRCA2 mutations remains to be determined. However, a decision analysis by Schrag et al. [1997] suggests that a 30-year-old woman with a BRCA1 or BRCA2 mutation may expect an increased life expectancy of 2.9–5.3 years from a mastectomy and 0.3–1.7 years from a prophylactic oophorectomy. Meijers-Heijboer et al. [2001] report a significant decline in breast cancer incidence in BRCA1 and BRCA2 mutation carriers who obtained a prophylactic mastectomy within 3–4 years of follow-up. Frost et al. [2000] found generally positive psychological and social responses to prophylactic mastectomy for the reduction of cancer risk. Utilization of prophylactic mastectomy may increase substantially if significant risk reduction is confirmed and this information is incorporated into counseling discussions.
We found that the majority of female mutation carriers (73%) discussed their results with their physician or health care provider. By the same token, however, 27% chose not to share this important health information. It is possible that concern over insurance or employment discrimination led some women to limit communication with physicians as a way of preventing their mutation status from entering their medical records, a risk specifically discussed with participants in our study. Non-carriers were less likely to discuss their results with physicians, although this may indicate that many personal physicians did not identify the high incidence of cancer in this kindred.
Our study is limited by the homogeneous character of our study population with respect to race and religion. In addition, some participants in this project were aware of their high-risk status prior to this project and 20% had been involved in other research projects to identify the BRCA1 gene. Additional research is necessary to characterize responses to genetic testing for breast and ovarian cancer susceptibility conducted in community practice settings and in other diverse populations and levels of baseline risk. In general, we are encouraged by the relatively high rates of adherence to screening recommendations following genetic testing. If this behavior pattern is maintained and if screening proves beneficial to BRCA1 mutation carriers in reducing morbidity and mortality from cancer, genetic testing will enhance the welfare of high-risk women.
Acknowledgements
We would like to thank Elizabeth Thomson, RN from the Ethical, Legal and Social Implications Branch of the NHGRI and Susan Nayfield, M.D. of the NCI for their support in this project. Colleagues who were invaluable to the project include: David Goldgar, Corinne Halls, Diana Lane, Georgia Hatch, Tamra Frei, Linda Steele, Bonnie Flick, MD, Melanee Kilpack, Teri Dickert, Sara Taub, Tim Ma, Nick Cassavaugh, Jennifer West, Heather DeMuth, Rachel Barnes, Rebecca McTee, Leslie DiMella, Shanda Breckenridge, Danielle Cutler, Bryan Warnick, Ted Johnson, Emily Wilcox, Rob Stoll, Tony Werrett, and Emma Milivogevic.




