Deletion of 11q24.2-qter with agenesis of unilateral internal carotid artery and total anomalous pulmonary venous return
To the Editor:
Congenital absence of the internal carotid artery (ICA) is an extremely rare anomaly [Fisher, 1913]. Partial monosomy for 11q, del(11q), is also a rare chromosome abnormality, and about 50 cases of del(11q) are known in the literature [Jacobsen et al., 1973; Kaffe et al., 1977; Laurent et al., 1979; McPherson and Meissner, 1982; O'Hare et al., 1984; Fryns et al., 1986; Voullaire et al., 1987; Schwarz et al., 1992; Hustinx et al., 1993; Lewanda et al., 1995; Penny et al., 1995]. There has been no report on the agenesis of the ICA associated with del(11q). Here we present an association of the two conditions which may provide some clues to defining their possible, causal relationship.
The patient, a Taiwanese boy, was the term-product of a 31-year-old G2-P2 mother and a nonconsanguineous 31-year-old father. His mother suffered from preeclampsia and gestational diabetes mellitus during pregnancy. There was no family history of any inherited diseases. Due to malposition, the baby was born by cesarean section at a local hospital, with a birth weight of 2,280 g (< 3rd percentile), length of 40.6 cm (< 3rd percentile), and occipitofrontal circumference of 31 cm (< 3rd percentile). Intrauterinal growth retardation and placental insufficiency were noted at the 30th week of gestation. In addition to the small size for gestational age, frontal bossing, brachydactyly of the right foot, syndactyly of the left foot, and thrombocytopenia (62,000/mm3) without hepatosplenomegaly were also noted at his birth. Because the patient had feeding cyanosis and tachypnea without heart murmur, he was transferred to our hospital 2 days post-delivery. Physical examinations at 1 month of age revealed trigonocephaly, bilateral ptosis of eyelids, left-eye coloboma, hypertelorism, cyanotic lips, tachypnea, subcostal retraction, clenched hands, brachydactyly with an absent fifth toe of the right foot, and syndactyly of toes 4 and 5 of the left foot (Fig. 1A–C). There were no apparent hypotonia, hypertonia, or other focal neurological signs. Echocardiography revealed a patent ductus arteriosus, atrial septal defect, and total anomalous pulmonary venous return (infracardiac type). The patient received a total correction of the heart at 12 days of age. Chromosome analysis revealed 46,XY,del(11)(q24.2-qter) (Fig. 1D). Karyotypes of the parents were normal. Transcranial Doppler study revealed increased pulse indexes in the left middle cerebral artery (MCA), bilateral posterior cerebral artery (PCA), and right anterior cerebral artery (ACA), and reversed flow in the left ACA. Brain magnetic resonance angiography (MRA) demonstrated the absence of the left ICA (Fig. 2A). Computed tomography (CT) of the temporal bone demonstrated agenesis of the left carotid canal (Fig. 2B). A brain single-photon emission CT (SPECT) scan with [99mTc] HMPAO revealed rather symmetrical but diffusely-decreased perfusion to the bilateral cerebral and cerebellar hemispheres (Fig. 2C). At age 5 months he suffered from aspiration pneumonia with acute respiratory distress syndrome, and died in May, 2000.
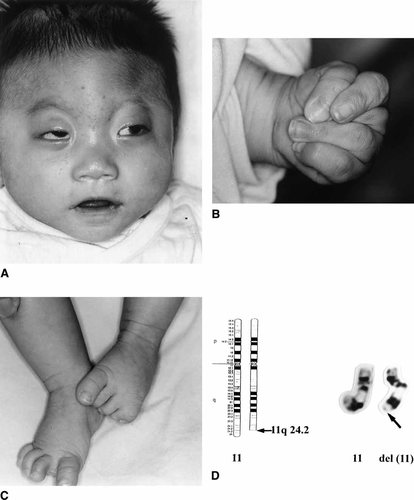
Patient at age 4 months. A: Note the trigonocephaly, hypertelorism, bilateral potosis, depressed nasal bridge, and micrognathia. B: Clenched hand of the left side. C: Brachydactyly with an absent fifth toe of the right foot and syndactyly of toes 4 and 5 of the left foot. D: Normal and del(11) chromosomes with a breakpoint at 11q24.2 (arrow).
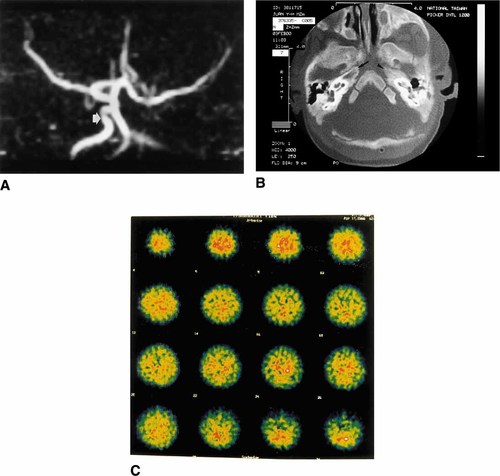
A: MRA of the brain showing no flow signal in the left ICA, compared with the disclosed flow signal in the right ICA (arrowhead). B: Axial CT through the skull base demonstrating the absence of the left carotid canal (arrowhead) and the normal right canal (arrow). C: [99m Tc] HMPAO perfusion SPECT scan (transaxial view) showing diffusely decreased perfusion in the bilateral cerebral and cerebellar hemispheres. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
This patient had de novo deletion of 11q24.2-qter, the agenesis of the ICA, and total anomalous pulmonary venous return. This association has not been described in 50 reported cases of such chromosomal deletion [Lewanda et al., 1995]. A review of the 50 previous cases revealed that the chance of an associated cardiac anomaly was greater than 50%. These include VSD, hypoplastic aorta, interrupted aortic arch, tetralogy of Fallot, truncus arteriosus [Jacobsen et al., 1973; McPherson and Meissner, 1982], hypoplastic left heart syndrome [Kaffe et al., 1977; Voullaire et al., 1987; Hustinx et al., 1993], and coarctation of the aorta [Fryns et al., 1986]. However, neither total anomalous pulmonary venous return nor agenesis of the ICA, as seen in our patient, have been previously reported in any cardiac defects attributable to 11q deletion. The only CNS defects that were associated with 11q deletion included an abnormality in the supratentorial white matter [Wardinsky et al., 1990] and hypogenesis of the corpus callosum [Owada and Sakuta, 1995]. The thrombocytopenia noted in our patient did not improve until his last admission. As thrombocytopenia, anemia, neutropenia, and congenital heart diseases were relatively common features in cases of 11q-deletion [Lewanda et al., 1995], certain genes or loci (such as the Ets-1 and nuclear-factor-related-kB (NFRKB) genes [Penny et al., 1995]) at the distal region of 11q may influence hematopoiesis. It remains to be determined whether a deletion in the distal region of 11q plays a role in the development of the cardiovascular system.
Acknowledgements
We thank the deceased's parents for consenting to this article. We also thank Formosa Medical Editors (FME) for the revision of the manuscript.




