Homozygous α-thalassemia associated with hypospadias: SEA-type deletion does not affect expression of the -14 gene and loss of the θ1-globin gene on 16p13.3 is compensated by its duplicate θ2 on chromosome 10
To the Editor:
Homozygous α-thalassemia of the Southeast Asian (SEA)-type has been observed in association with hypospadias in eight male survivors [Dame et al., 1999a; Fung et al., 1999]. Females with homozygous α-thalassemia (- -SEA/- -SEA) or the heterozygous state (- -SEA/α α) or HbH (- -/- α) disease show normal genitalia. These findings suggest a common etiology for both entities favoring a recessive trait involved only in the development of male genitalia. Recently, we speculated whether the SEA-deletion may either generate a new gene product originating from the SEA-breakpoint on chromosome 16p13.3 (Fig. 1) or lead to a imbalance in transcription of the -14 gene that harbors the α-globin gene promoter (HS-40) in its intron 5 [Dame et al., 1999b]. Alternatively, the θ1-globin gene may encode an unknown factor and mutations herein could interfere with genital ontogeny.
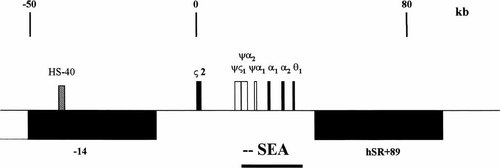
Genomic organization around the α-globin cluster on chromosome 16p13.3 with coordinates (given in kb) shown with respect to the ζ2-globin mRNA cap site (position 0) [according to Flint et al., 1997]. The genomic extent of widely expressed genes (black boxes) and pseudogenes (open boxes) are depicted with their direction of transcription either to the centromeric (above the line), or to the telomeric region (below the line). The HS-40 element (located in intron 5 of the partially outlined -14 gene) is shown by a hatched box and the extent of the SEA-deletion is indicated by a horizontal line; hSR+89 indicates a novel gene discovered most recently (Utsch et al., submitted for publication).
To test these hypothesizes we investigated DNA and RNA samples obtained from our index patient described previously [Dame et al., 1999a]. First, we looked for a cryptic gene product that may arise due to an unknown transcription factor occupying the novel palindromic sequence (GGAGGTTCACTTGGAGG) created at the SEA-deletion breakpoint [Nicholls et al., 1987]. Various approaches using different candidate primers, however, failed to detect a cryptic mRNA, and these findings were supported by the GRAIL2 (v1.3) exon prediction program that was unable to identify potential exons located around the SEA-breakpoint.
Given this, we focussed our attention on the expression of the -14 gene. The major regulatory element of the α-globin gene cluster (HS-40) is located in intron 5 of the -14 gene (Fig. 1) [Vyas et al., 1995]. Therefore, the SEA-type deletion may interfere with its transcription, probably due to continuous occupation by the globin-specific transcription apparatus. The resulting imbalance in the expression of the functional unknown -14 gene may contribute to the formation of hypospadias. The quantity of RT-PCR products covering several parts of the -14 cDNA from our SEA-male survivor, however, were comparable with the amounts detected in normal control.
The θ1-globin transcript was initially detected only in fetal liver, fetal yolk sac, erythroid tissues and in the erythroleukemic cell line FK562 [Leung et al., 1987; Hsu et al., 1988] and trace amounts were subsequently detected in reticulocytes and adult bone marrow cells [Mamalaki et al., 1990]. Using non-nested RT-PCR, we observed its expression in an apparently sex- and age-independent manner in all adult tissues tested (leukocytes, kidney, adrenal gland, placenta, liver, skin, white and grey matter of the temporal lobe) and 15 unrelated cases with isolated hypospadias were analyzed for mutations in this gene. All these patients had been examined for other so far known factors attributable to hypospadias according to a standardized set of diagnostics [Albers et al., 1996]. Besides a single heterozygous Pro115(CCC)-to-Ala(GCC) change found in one patient, all other samples revealed a complete θ1-globin genomic sequence indistinguishable from normal.
A striking observation was that both, a θ1 genomic fragment and the full-length θ1-globin-cDNA could be amplified from independent blood samples obtained from our index patient. Sequencing of the genomic fragment and the reverse transcribed PCR-product revealed an intact θ1 coding sequence, thereby questioning our initial diagnosis [Dame et al., 1999a]. Investigation of the SEA-deletion breakpoint using primers according to Chang et al. [1991], however, showed unequivocally the heterozygous junction sequence in both the parents and its homozygous state in the patient. Moreover, a female survivor afflicted with homozygous SEA-α-thalassemia was also shown to express the θ1-globin gene. Given this, a second θ-globin gene should reside elsewhere in the genome. These findings were supported by sequence data most recently deposited in GenBank (Smith, DR; unpublished; accession number AC069076). Here, the complete theta-globin gene and more than 3 kb of flanking sequences were shown to be part of chromosome 10. In contrast to these sequence data implying a frameshift and a nucleotide transition in the coding sequence (ΔT117 and C137→T, nucleotide numbering starting with 1 in exon 2), however, we found the correct message in the cDNAs of both patients. For this reason, an intact θ2-globin should be transcribed from this region.
These results imply that neither the genes encoding θ-globin or the -14 protein nor the newly discovered hSR+89 gene [Utsch et al., submitted] that terminates proximal (4.3 kb) to the SEA-breakpoint seem to be involved in the formation of SEA-related hypospadias.
Acknowledgements
We thank R. Schubert for technical assistance.




