FGFs, their receptors, and human limb malformations: Clinical and molecular correlations†
The first two authors contributed equally to this work.
Abstract
Fibroblast growth factors (FGFs) comprise a family of 22 distinct proteins with pleiotropic signaling functions in development and homeostasis. These functions are mediated principally by four fibroblast growth factor receptors (FGFRs), members of the receptor tyrosine kinase family, with heparin glycosaminoglycan as an important cofactor. Developmental studies in chick and mouse highlight the critical role of FGF-receptor signaling in multiple phases of limb development, including the positioning of the limb buds, the maintenance of limb bud outgrowth, the detailed patterning of the limb elements, and the growth of the long bones. Corroborating these important roles, mutations of two members of the FGFR family (FGFR1 and FGFR2) are associated with human disorders of limb patterning; in addition, mutations of FGFR3 and FGF23 affect growth of the limb bones. Analysis of FGFR2 mutations in particular reveals a complex pattern of genotype/phenotype correlation, which will be reviewed in detail. Circumstantial evidence suggests that the more severe patterning abnormalities are mediated by illegitimate paracrine signaling in the mesoderm, mediated by FGF10 or by a related FGF, and this is beginning to gain some experimental support. A further test of this hypothesis is provided by a unique family segregating two FGFR2 mutations in cis (S252L; A315S), in which severe syndactyly occurs in the absence of the craniosynostosis that typically accompanies FGFR2 mutations. © 2002 Wiley-Liss, Inc.
INTRODUCTION TO FIBROBLAST GROWTH FACTORS AND THEIR RECEPTORS
Fibroblast growth factors (FGFs) were initially defined in the mid-1970s as mitogenic and angiogenic activities in brain extracts. Since that time, further members of the family have been progressively identified, initially by experimental means and more recently backed up by bioinformatic approaches. The family comprises 22 known members in humans (reviewed by Ornitz and Itoh [2001]). FGFs show considerable variation in their primary sequence, especially at the N- and C-termini, but are predicted to share a similar trefoil core structure comprised of three β-sheets of four strands each.
The first FGF receptor (FGFR) was identified in the chick by affinity purification of proteins bound to FGF2, followed by amino acid sequencing [Lee et al., 1989]; four distinct FGFRs (numbered 1–4) are present in vertebrates. FGFRs are transmembrane proteins belonging to the receptor tyrosine kinase (RTK) family. They are characterized by three extracellular immunoglobulin (Ig)-like domains, a single-pass transmembrane segment, and a split tyrosine kinase (TK) domain. Signaling occurs following the dimerization of two FGFR molecules, which is promoted by the binding of two FGF molecules to the extracellular Ig domains, forming a tetrameric complex. Cell surface heparan sulfate proteoglycans are important cofactors in this activity (reviewed by Ornitz [2000]). Although three crystal structures of the binding complex have been obtained by independent groups, the details remain controversial [Pellegrini et al., 2000; Plotnikov et al., 2000; Stauber et al., 2000].
Additional complexity in FGF-receptor signaling is achieved by a wide array of alternative splicing choices (reviewed by Johnson and Williams [1993]). Most importantly, in the case of FGFR1, FGFR2, and FGFR3, an obligatory alternative splicing event generates two forms of the IgIII domain with different FGF binding characteristics [Ornitz et al., 1996]. The details are best established in the case of FGFR2, for which the alternatively spliced forms [IgIIIa/IIIb, termed FGFR2b or keratinocyte growth factor receptor (KGFR), and IgIIIa/IIIc, termed FGFR2c or BEK] show striking differences in binding affinity for many FGFs (Fig. 1). The cell specificity of alternative splicing appears to be controlled by competition between the IIIb and IIIc exons for splicing factors, which depends on the intrinsic sequence of the exons and introns and is modulated by the binding of tissue-specific trans-acting factors to defined sequence elements [Del Gatto et al., 1997; Carstens et al., 1998; Le Guiner et al., 2001]. As discussed later, this alternative splicing mechanism appears to play an important role in the occurrence of FGFR2-mediated limb malformations.
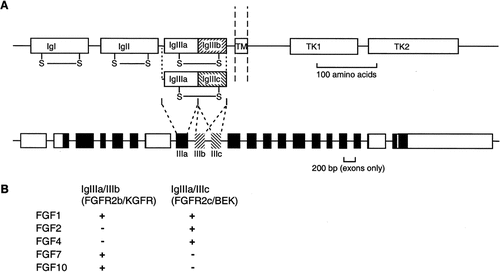
Structure and alternative splicing of FGFR2. A: Diagram of domain structure (above) showing immunoglobulin-like domains I (IgI), II (IgII), and the alternatively spliced IIIa/IIIb or IIIa/IIIc (IgIIIa/IgIIIb and IgIIIa/IgIIIc, respectively), each of which contains an intramolecular disulfide bridge (-S-S-), transmembrane segment (TM), and split tyrosine kinase domain (TK1, TK2). The exon structure below shows noncoding regions (white boxes) and coding regions (black boxes). The alternatively spliced exons IIIb and IIIc are hatched in two different patterns. B: Binding of the FGFR2b and FGFR2c alternative spliceforms to a selection of FGF ligands.
FGFR dimerization promotes the intracellular trans-autophosphorylation of critical tyrosine residues in the activation loop of the receptor; this stabilizes the TK domain in an active conformation leading to further phosphotyrosine formation at other tyrosine residues in cis within the TK domain, which serve as targets for the binding of proteins such as SHC, FRS2, and PLCγ. Insight into these processes is provided by the structure of the FGFR1 TK domain solved by Mohammadi et al. [1996]. Additional FGF receptors have been identified, such as a cysteine-rich receptor [Köhl et al., 2000] and a truncated version of an FGFR [Wiedemann and Trueb, 2000], but their physiological roles are uncertain. The cellular consequences of FGFR activation are diverse and may affect cell division, differentiation, or death according to the context. From the perspective of morphogenesis, effects on cell migration and on epithelial-mesenchymal interaction are particularly important.
FGF SIGNALING IN LIMBS
The critical role of FGF signaling in the limbs was highlighted in the study by Niswander and Martin [1993], which demonstrated that the defect in limb outgrowth caused by removal of the apical ectodermal ridge (AER) could be partially rescued by replacement with a bead soaked in FGF4. Since then, evidence has steadily accrued that FGF signaling is important at almost every stage of limb development, including the positioning of the limb buds, the maintenance of limb outgrowth, the detailed patterning of the limb elements, and the growth of the long bones. An outline of these critical FGF-mediated processes is summarized below; the reader is referred to reviews by Martin [1998] and Tickle and Münsterberg [2001] for further details. It is important to note that our current understanding of the role of FGFs in limb development is likely to be far from complete. Little is known about the physiological role of several FGFs that have been discovered relatively recently using bioinformatic means. In addition, owing to the cross-reactivity of FGFs and their overlapping expression patterns and receptor specificities, a developmental response to an FGF-soaked bead (a commonly used means of experimental analysis) tells one that an FGF-limited signaling process is occurring, but does not indicate that the particular FGF used is physiologically important in that context. The interpretation of genetic knockout experiments is also hampered by functional redundancy. With these caveats, key FGF-mediated signaling processes in the limb are summarized below.
Position of limb outgrowth
Experiments by Cohn et al. [1995] and Ohuchi et al. [1995] originally demonstrated that the entire flank has the capacity to generate ectopic limbs if stimulated by FGF at the appropriate time. The appearance of fore- and hindlimbs at their correct positions is believed to be initiated by a pulse of FGF8 signaling from the intermediate mesoderm, which induces FGF10 expression in the adjacent lateral plate mesoderm.
Establishment and maintenance of limb outgrowth
Signaling by FGF10 in the mesoderm in turn induces FGF8 expression in the AER, an antero-posteriorly orientated band of specialized epithelium at the tip of the limb bud. Significantly, these FGFs bind to different receptors (FGF10 exclusively to FGFR2b, FGF8 to FGFR2c), which are expressed in patterns complementary to their cognate factors (FGFR2b expression is predominantly epithelial, FGFR2c expression is mesenchymal). This sets up a feedback loop of epithelial-mesenchymal induction. Recent evidence suggests that several molecules belonging to the WNT family mediate these reciprocal events [Kawakami et al., 2001]. The importance of this feedback loop is illustrated by the absence of limbs in mice homozygous for targeted null mutations of either Fgf10 or the FGFR2b isoform of Fgfr2 [Min et al., 1998; Sekine et al., 1999; De Moerlooze et al., 2000; Revest et al., 2001] and the underdeveloped limbs in mice with a limb-specific knockout of Fgf8 [Lewandoski et al., 2000; Moon and Capecchi, 2000]. Signal transduction involves activation of protein kinase C [Lu et al., 2001]. Once outgrowth is established, expression of FGFR1c in the mesenchyme below the AER (termed the progress zone) is essential to maintain reciprocal signaling [Xu et al., 1999; Revest et al., 2001].
Anterior-posterior patterning of the limb
In contrast to FGF8, FGF4 expression is restricted to the posterior two-thirds of the AER. Maintenance of sonic hedgehog-mediated polarizing activity in the posterior part of the limb was thought to depend on continued FGF4 signaling. However, recent studies of mice with limb-specific knockout of FGF4 activity, which have normal limbs, favor a model in which a combination of FGFs (candidates include FGF9 and FGF17) fulfills this function [Moon et al., 2000; Sun et al., 2000].
Patterning of individual skeletal elements and interdigital apoptosis
Human limb malformations caused by FGFR mutations provide evidence that the patterning of individual skeletal elements is FGF-dependent. The details of this process are complex and poorly understood. In the human, FGFR1 and FGFR2 are expressed in condensing mesenchyme but later become localized to the perichondrium, with strong articular expression of FGFR2; FGFR3 is upregulated in proliferating growth plate chondrocytes [Delezoide et al., 1998; Wang et al., 2001]. In the chick, FGF beads implanted in the interdigital mesoderm initially inhibit but later promote cell death between the digits [Montero et al., 2001].
Outgrowth of the limbs by endochondral ossification
Once limb pattern is established, longitudinal growth is achieved by a highly ordered process of cell division and differentiation at the epiphyseal plates. An FGFR3-mediated signaling process is critical for this, as illustrated both by the phenotypes of Fgfr3−/− mice, which have excessive long bone growth, and by human bone dysplasias caused by activating FGFR3 mutations (reviewed by Naski and Ornitz [1999] and Vajo et al. [2000]). FGF18 is a strong candidate as the physiological ligand for this process [Liu et al., 2002; Ohbayashi et al., 2002].
MUTATIONS OF FGF/FGFR PATHWAYS THAT CAUSE LIMB DISORDERS IN HUMANS
Spectrum of Mutations
The intimate involvement of FGF-signaling in limb development is attested to by the fact that abnormalities of limb patterning or growth are observed in association with mutations of three of the four canonical FGFRs (Table I). Somewhat paradoxically, mutations of only one FGF, FGF23, are known to be associated with abnormal limb development in humans, in the disorder autosomal dominant hypophosphatemic rickets [ADHR Consortium, 2000]. The relative lack of mutations in FGFs probably reflects a combination of factors. Some FGFs may have essential embryonic roles, whereas the importance of others is masked by functional redundancy. Mutations of FGFs are more likely to cause recessive loss-of-function phenotypes, in contrast to the gain-of-function FGFR mutations: these may be more difficult to map genetically. Moreover, many FGFs have been identified too recently for their potential role in human disorders to have been adequately investigated.
| Gene | Phenotype | Principal limb abnormalities | Key references |
|---|---|---|---|
| FGF23 | Autosomal dominant hypophosphatemic rickets | Rickets, osteomalacia, lower limb deformity |
ADHR Consortium [2000] |
| FGFR1 | Pfeiffer syndrome | Broad first digits, fused phalanges, cutaneous syndactyly |
Roscioli et al. [2000] |
| FGFR2 | Apert syndrome | Broad first digits, complex bony syndactyly, symphalangisms radio-humeral or -ulnar synostosis | |
| Pfeiffer syndrome | Varies from broad hallux only, sometimes also broad thumb, symphalangism, cutaneous syndactyly, radio-humeral or -ulnar synostosis |
See Table II |
|
| Beare-Stevenson syndrome | Broad first digits |
Krepelová et al. [1998] |
|
| FGFR3 | Achondroplasia | Proximal limb shortening, tibial bowing, limited elbow extension, trident hand |
Hunter et al. [1998] |
| Hypochondroplasia | Mild limb shortening |
Ramaswami et al. [1998] |
|
| Thanatophoric dysplasia | Severe limb shortening and bowing |
Wilcox et al. [1998] |
|
| SADDAN syndrome | Severe limb shortening and bowing |
Bellus et al. [1999] |
Among the mutations featured in Table I, those involving FGFR2 are the most interesting to the aficionado of limb development. The FGFR3 and FGF23 mutations primarily reflect abnormalities of bone growth that are not restricted to the limbs; this is really a separate field of investigation and will not be discussed further here. The case of the single FGFR1 mutation (which encodes the amino acid substitution P252R and causes a mild form of Pfeiffer syndrome) is intriguing. Originally discovered by Muenke et al. [1994], rather little progress has been made in understanding either the human genetics or the developmental mechanism since then (reviewed by Roscioli et al. [2000]). A mouse model of this mutation was recently constructed, but the limbs were reported as normal [Zhou et al., 2000]. At present, little can be said about the cause of the abnormal limb pattern in FGFR1 mutation-associated Pfeiffer syndrome.
Examination of FGFR2 reveals a much greater richness of limb phenotypes that are proven, or suspected, to be associated with abnormal limb patterning, and this will be the focus of the remainder of this work. Over the past 8 years, many FGFR2 mutations have been described, mostly in association with one or other of three syndromic diagnoses: Crouzon, Pfeiffer, or Apert syndrome. All three syndromes are characterized by craniosynostosis, but are distinguished by the respective limb phenotype. Traditionally speaking, in Crouzon syndrome the limbs are normal, in Pfeiffer syndrome the thumbs and halluces are broad, and in Apert syndrome there is a complex bony syndactyly involving, at a minimum, the central three digits. It is essential to recognize that the distinction between these three syndromes is not absolute, but categorizes a complex, multidimensional gradation of phenotypes into three convenient labels. In Crouzon syndrome, for example, radiological investigation of the hands and feet frequently reveals minor abnormalities that are not evident on clinical examination [Murdoch-Kinch and Ward, 1997]. In Pfeiffer syndrome, the severity of broadening and medial deviation of the first digital ray varies widely; sometimes only the halluces are broad, a pattern recognized by some clinicians as Jackson-Weiss syndrome. (In our view, such phenotypic splitting is not helpful and is likely to lead to confusion; see Cohen [2001].) Even Apert syndrome may show occasional overlap with Pfeiffer syndrome, as illustrated by the cases reported by Passos-Bueno et al. [1997, 1998].
How can we sort through this complexity? The first task is to establish the data set. Fortunately, the application of FGFR2 mutation analysis to patients with craniosynostosis and limb anomalies has identified some clear trends in genotype-phenotype correlation for the most frequent mutations, which are summarized in Table II and Figure 2. Two important patterns emerged from the early observations [Wilkie, 1997]. First, mutations localized to either one of just two amino acids (S252, P253) in the linker between the IgII and IgIII domains cause nearly all cases of Apert syndrome; second, whereas mutations scattered throughout the body of the IgIIIa and IgIIIc domains cause either Crouzon or Pfeiffer syndrome (although most mutations tend to be preferentially associated with one or other phenotype), mutations of the splice sites surrounding the alternatively spliced IIIc exon are mostly associated with Pfeiffer syndrome.
| Mutation | Diagnosis (% of FGFR2-positive cases of syndrome) | Limb phenotype | Other comments | Key references |
|---|---|---|---|---|
| S252W | Apert syndrome (66%) | Syndactyly more commonly involving only three central digits | More severe craniofacial phenotype than P253R |
Slaney et al. [1996]; Lajeunie et al. [1999]; von Gernet et al. [2000] |
| P253R | Apert syndrome (33%) | Syndactyly more commonly including the thumb and hallux | Less severe craniofacial phenotype than S252W | As for S252W |
| C342R | Pfeiffer syndrome (∼20%) | Broad hallux, thumb often normal | Moderately severe craniofacial phenotype; the single most common cause of Pfeiffer syndrome | |
| W290C and S351C | Pfeiffer syndrome (∼20% combined) | Thumbs and halluces variably broadened; frequent radio-humeral or radio-ulnar synostosis may suggest a diagnosis of Antley-Bixler syndrome | Cloverleaf skull frequent, with ocular and airway compromise |
Schaefer et al. [1998]; Okajima et al. [1999]; Reardon et al. [2000]; Tsai et al. [2001] |
| Exon IIIc acceptor splice site | Pfeiffer syndrome (∼20% combined) | Broad, often medially deviated thumbs and halluces with cutaneous syndactyly and symphalangism, sometimes approaching the severity of Apert syndrome | Moderately severe craniofacial phenotype |
Schell et al. [1995]; Passos-Bueno et al. [1997]; Mulliken et al. [1999]; Oldridge et al. [1999] |
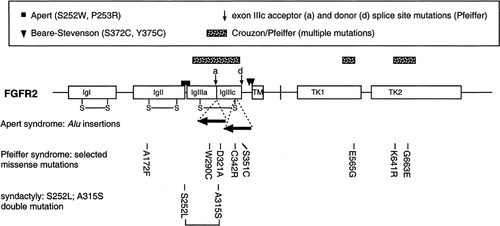
Overview of the distribution of mutations in FGFR2. Above a cartoon of the protein structure (annotated as in Fig. 1), the major patterns of genotype-phenotype correlation are summarized. Below are shown the positions of Alu insertions in Apert syndrome, specific missense mutations associated with Pfeiffer syndrome, and the double mutation identified in the family with isolated syndactyly.
Insights From Apert Syndrome
A link between these apparently disparate observations on Apert and Pfeiffer syndromes was made in an unexpected fashion. Two patients with clinically typical Apert syndrome who were negative for the canonical IgII-IgIII linker mutations were both found to harbor insertions of ∼350 bp Alu elements in the neighborhood of the IIIc exon. One of these occurred just upstream of the exon, the other was within the exon itself. We hypothesized that these insertions would interfere with the normal balance of exon IIIb/IIIc alternative splicing; to seek evidence for this, we cultured fibroblasts and keratinocytes obtained from a skin biopsy of one of these patients. We then demonstrated, by reverse transcriptase PCR, that the fibroblasts, which should normally express only the IIIc exon, also showed illegitimate expression of the IIIb exon [Oldridge et al., 1999]. This led us to examine the effect of two different IIIc acceptor splice site mutations associated with Pfeiffer syndrome, and these also showed illegitimate exon IIIb expression in fibroblasts, but at a lower level than in the Apert patient [Oldridge et al., 1999]. This latter observation has been independently confirmed in two further Pfeiffer syndrome patients [Tsukuno et al., 1999], although the interpretation that this represents haploinsufficiency is misguided [Johnson and Wilkie, 2000].
The significance of this work is that it provided the first cogent evidence that the limb abnormalities in Apert syndrome are caused by aberrant FGFR2b-type signaling in the mesenchyme. This contrasts with the situation for craniosynostosis, which must be predominantly mediated by abnormal FGFR2c signaling, because the missense mutations in exon IIIc that most commonly cause Crouzon syndrome do not affect the structure of the FGFR2b isoform. The implication that in Apert syndrome the abnormal developmental pathways for syndactyly (FGFR2b-type) and craniosynostosis (FGFR2c-type) might be distinct had resonance with previous observations made by Slaney et al. [1996]. This study, which compared the phenotypes associated with the two common Apert mutations, observed that whereas syndactyly was more severe in the case of the P253R mutation, cleft palate was more frequent with the S252W mutation, leading to the conclusion that “the mechanisms of signaling are not identical in the developing limb and skull” [Slaney et al., 1996]. These phenotypic findings were subsequently confirmed and extended by two other groups [Lajeunie et al., 1999; von Gernet et al., 2000].
To understand how this dissociation of skull and limb phenotypes might occur, biochemical studies are necessary to elucidate the effect of the canonical Apert mutations on the mutant protein. Anderson et al. [1998] examined the binding of various FGFs to mutant FGFR2c constructs and observed enhanced binding affinity for physiological FGF ligands. This was most marked for FGF2 and consistently greater for the S252W than the P253R mutation. This work implied that the Apert mutations conferred a gain-of-function by causing prolonged signaling. The greater effect for the S252W mutation in binding FGF2 (a selective FGFR2c ligand) would be in keeping with the craniosynostosis being an FGFR2c-mediated effect.
More recently, Yu et al. [2000] confirmed and extended these observations by demonstrating an additional effect of the Apert mutations: loss of ligand selectivity. Specifically, the S252W and P253R FGFR2c mutants, but not the wild-type protein, showed illegitimate binding to FGF7 and FGF10 (indicated by induction of reporter activity from an osteocalcin FGF response element, mitogenic response, and tyrosine phosphorylation of the kinase domain). These observations have striking parallels with those of Oldridge et al. [1999], suggesting that the Apert limb abnormalities occur as a result of paracrine activation of signaling by FGF7/10 or a related ligand within the mesenchyme, caused either by having an abnormal receptor in its normal place (Fig. 3B) or a normal receptor in the wrong place (Fig. 3C; reviewed by Yu and Ornitz [2001]). Moreover, the affinity of the P253R-FGFR2c mutant for FGF7 was only 1.5-fold lower than wild-type FGFR2b, whereas the S252W-FGFR2c mutant had a 24-fold lower affinity. This observation is in keeping with the greater severity of syndactyly associated with the P253R mutation [Slaney et al., 1996]. Yu et al. [2000] also observed alterations in the binding properties of FGFR2b mutant isoforms, the significance of which is currently unclear.
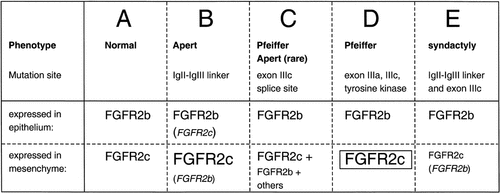
Altered FGFR2b and FGFR2c signaling proposed to give rise to limb malformations. Compared to normal signaling shown in A, different types of abnormal signaling are indicated as follows. Increased and decreased activity of the isoform is shown in larger- and smaller-case type, respectively; illegitimate activity of the isoform is shown in brackets and italics; ectopic expression of the isoform is shown with a plus sign and upright type; constitutive activation is boxed. B: Canonical Apert mutations. Enhanced FGF2/FGFR2c signaling causes craniosynostosis, illegitimate FGF7/FGF10 binding by the FGFR2c isoform causes syndactyly. The significance of the potential illegitimate binding by the FGFR2b isoform in the epithelium is unknown. C: Exon IIIc splicing mutations. Ectopic FGFR2b expression causes syndactyly, craniosynostosis may be caused by additional minor illegitimate spliceforms (see Johnson and Wilkie [2000]). D: The milder limb phenotypes in other Pfeiffer mutations may be caused by constitutive activation of FGFR2c. E: Proposed mechanism of the double-mutation S252L; A315S. FGFR2c isoform has weaker native activity but binds illegitimately to FGF7/FGF10 or a related ligand.
In an extension of this work, Ibrahimi et al. [2001] compared the wild-type and Apert mutant crystal structures of FGFR2c bound to FGF2, finding evidence that the Apert mutations act by making additional contacts with FGF. Surprisingly, the two mutations make a completely different set of contacts. Those made by the S252W mutation lie outside the conserved FGF core and therefore enhanced binding is only expected to a limited subset of FGFs (including FGF2 and FGF7). By contrast, the P253R mutation makes additional contacts within the conserved FGF core, predicting that its enhanced FGF-binding effect will be promiscuous for most FGFs. If these mechanisms are correct, it is surprising that the phenotypes attributable to the two mutations are as similar as is actually observed. This suggests that the pathological effects are mediated by only a limited repertoire (perhaps as few as two) of the FGF ligands.
As a postscript to this discussion, it should be noted that a radically different mechanism of FGFR activation has been proposed, based on the crystal structure of FGF1 bound to FGFR2 obtained by another research group [Pellegrini et al., 2000]. The key difference between the structures revolves on whether the P253 residue (mutated in Apert syndrome) adopts a cis- or trans-conformation. Clearly, explaining the behavior of Apert mutations will lie at the heart of finding the correct model of FGFR function.
Mechanisms of Pfeiffer Syndrome
As well as the exon IIIc splice site mutations described above, Pfeiffer syndrome may be caused by a wide variety of missense mutations encoded by the IIIa and IIIc exons of FGFR2 (characteristic mutations are summarized in Fig. 2 and Table II). Many of these mutations either substitute one of the paired cysteine residues (at C278 and C342) or create an extra cysteine (for example, W290C and S351C). The net result of both kinds of mutation is to produce an odd number of cysteine residues in the IgIII domain. As well as causing unfolding of the protein, these free cysteines enable mutant FGFR2 molecules to form covalently cross-linked dimers, resulting in constitutive activation [Neilson and Friesel, 1995; Robertson et al., 1998]. In those cases where the mutation occurs in exon IIIc, only the FGFR2c spliceform is mutant, leading to the conclusion that broadening of the first digit can also be mediated by FGFR2c (Fig. 3D). However, the severity of the abnormalities in the hands and feet is usually milder than in cases with splice site mutations (Table II). The few exceptions, such as the patient with extensive syndactyly illustrated by Nagase et al. [1998], in whom the authors found a D321A mutation (encoded by exon IIIc), may affect ligand-binding specificity [Plotnikov et al., 2000].
To gain further insight into the mechanisms of limb abnormality associated with FGFR2 mutations, we studied five patients in whom no mutation had been found in either exons IIIa or IIIc. Four of these patients had Pfeiffer syndrome, including two families apparently linked to FGFR2 [Schell et al., 1995]. The fifth patient had Philadelphia craniosynostosis, a unique entity characterized by Apert-like syndactyly associated with sagittal synostosis [Robin et al., 1996], also probably linked to FGFR2 [Yoshiura et al., 1997]. Our first hypothesis was that these patients might have mutations in one of the introns flanking exons IIIb and IIIc, which contain elements that are important for the control of alternative splicing [Del Gatto et al., 1997; Carstens et al., 1998]. However, complete sequencing of the region between exon IIIa and exon 10 (encoding the transmembrane region) revealed only a few single nucleotide polymorphisms. We undertook long-range PCR experiments that included these polymorphic residues, followed by restriction digestion, to exclude the possibility that one of the FGFR2 alleles was deleted for part or all of this region in any of these patients (data not shown).
Having failed to identify a causative genetic lesion, we examined the entire FGFR2 coding region by denaturing high-performance liquid chromatography (DHPLC) and identified a likely causative mutation in all four Pfeiffer syndrome patients, but not in the patient with Philadelphia craniosynostosis [Kan et al., 2002]. Three Pfeiffer syndrome patients each had a different mutation in the TK domain (E565G, K641R, G663E), in every case associated with distinct, but relatively mild, broadening of the thumbs and halluces. We have subsequently identified three other mutations in this region, also in patients with craniosynostosis and a “crouzonoid” facial appearance, but not necessarily with broad first digits. Two of the other mutations, N549H and K659N, occur at residues exactly equivalent to constitutively activating mutations in FGFR3 that cause hypochondroplasia, SADDAN syndrome, and thanatophoric dysplasia type 2 [Raffioni et al., 1998; Bellus et al., 2000], suggesting that the FGFR2 mutations are also activating.
The observation that mild broadening of the first digit is a variable consequence of FGFR2 mutations in the TK as well as the IgIII domain supports the impression that this phenotype arises as a rather nonspecific consequence of FGFR2 activation (Fig. 3D). It should be noted that at a developmental level, broadening of the first digital ray is likely to reflect a very subtle quantitative process—control of the length of the AER—which, at a human genetic level, is obscured by the dichotomous classification into Crouzon and Pfeiffer syndromes. It should therefore not come as too much of a surprise that in the case of some mutations (especially at C342), the eventual phenotype may fall to either side of a “broad digit” threshold [Rutland et al., 1995; Hollway et al., 1997], apparently giving rise to two different syndromes. Instead of the need to invoke special causal mechanisms, all that is being observed is the variation of a quantitative trait that depends on the combination of the specific FGFR2 mutation, unidentified factors in the genetic background and environment, and (given the usually symmetrical appearance of the associated limb malformations) probably only a small element of chance.
The phenotype in the final Pfeiffer syndrome family was more interesting. This family, the same as the original pedigree described by Pfeiffer [1964], from whom the syndrome derives its eponymous title, has a rather severe limb phenotype with very broad, medially angulated first digits, cutaneous syndactly of digits 2–5, brachydactyly, and absent middle phalanges in the feet with occasional symphalangism. This family has the first mutation of the IgII domain described for any FGFR—A172F [Kan et al., 2002]. The mutated alanine occupies a critical position at the turn between two strands of β-sheet and is also normally involved in a receptor:receptor contact according to the crystal structure of FGF2 bound to FGFR1 described by Plotnikov et al. [1999]. Although substitution to bulky phenylalanine might simply disrupt protein folding, the complex limb phenotype and the rarity of the association of IgII domain mutations with craniosynostosis hint at a more specific mechanism. One possibility is that stacking of the mutant phenylalanine side chains might strengthen the receptor:receptor contact in the tetrameric FGF:FGFR2 complex.
Limb Malformation as a Primary Phenotype Associated With FGFR2 Mutation
In all the cases referred to so far, craniosynostosis has tended to be the major presenting feature of the FGFR2 mutation, with the limb abnormalities taking a somewhat secondary place as a valuable means of differential diagnosis (although requiring plastic surgery in more severe cases). Recently, however, we have encountered a remarkable family with a double mutation of FGFR2, in which an Apert-like syndactyly was the presenting feature and craniosynostosis was absent.
The pedigree is shown in Figure 4. The proband III-4, a 1-year-old girl, and her mother II-9 were referred for genetic assessment because of syndactyly of the hands and feet. Both had a very similar pattern of syndactyly in all four extremities with broad, separate, and medially deviated first digits, partially separate fifth digits, and complete cutaneous syndactyly of the central three digits (Fig. 5A); a hand radiograph of the proband showed a single phalanx in the thumb and symphalangism of the proximal and middle phalanges of digits 2–5 (Fig. 5B). Apart from a rather broad forehead, the craniofacial appearance was normal (Fig. 5C); a skull radiograph of III-4 at the age of 3 years showed a wide sagittal suture and no evidence of craniosynostosis.
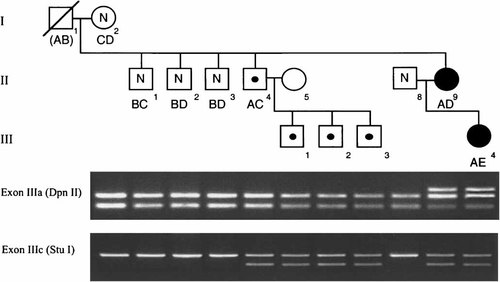
Identification of heterozygous S252L and A315S mutations of FGFR2 in a family with syndactyly. The pedigree shows the segregation of the syndactyly phenotype and S252L; A315S double mutation (filled shapes), asymptomatic carriers of the A315S mutation (central dots), and individuals negative for both mutations (N). Reconstruction of 10q26 haplotypes A–E, based on the microsatellite markers D10S190, D10S209, and D10S216, is shown below the corresponding pedigree symbol. The lower panel shows the pattern of restriction digestion of PCR-amplified fragments encompassing FGFR2 exons IIIa (digested with DpnII) and IIIc (digested with StuI).
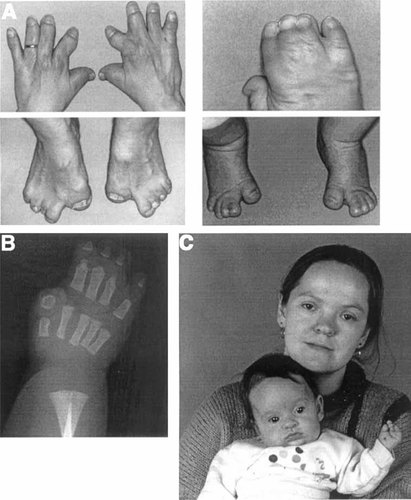
Phenotype associated with the heterozygous S252L; A315S mutation in FGFR2. A: Appearance of hands and feet in the mother II-9 (left; hands are postoperative) and daughter III-4 (right). B: Radiograph of right hand of III-4 in the neonatal period. C: Facial appearance of II-9 and III-4.
Surprisingly, single-strand conformation polymorphism analysis of exons IIIa and IIIc of FGFR2 revealed abnormal migration in both amplified fragments, and when these were sequenced, both the proband and her mother had two separate heterozygous mutations: 755C → T in exon IIIa, encoding the substitution S252L, and 943G → T in exon IIIc, encoding A315S. The mutations must be present in cis on the same FGFR2 allele, because both were transmitted from the mother to the daughter. Further investigation of the mother's family showed that although her own mother (I-2) and three of her brothers were negative for both mutations, a fourth brother II-4 was heterozygous for the A315S mutation but negative for the S252L mutation. Haplotyping with microsatellites that map close to FGFR2 on 10q26 showed that II-4 and II-9 had inherited, from their deceased father I-1, the opposite 10q26 haplotype to the three other brothers tested (Fig. 4). All three sons of II-4 also inherited the A315S mutation. We conclude that the deceased asymptomatic grandfather I-1 (who was aged 49 years when II-9 was born) was also heterozygous for the A315S mutation; he transmitted this mutant allele to his daughter, but in addition a second mutation, S252L, arose on this allele, revealing the syndactyly phenotype.
One of the instructive features of this scenario is that the phenotypes of both single FGFR2 mutations have been described previously, the S252L mutation by Oldridge et al. [1997] and the A315S mutation by Johnson et al. [2000]. Both mutations were associated with low-penetrance craniosynostosis but no syndactyly. The observation that none of the four individuals (II-4, III-1, III-2, III-3) heterozygous only for the A315S mutation had significant clinical problems provides valuable corroboration for the conclusion of Johnson et al. [2000] that this mutation is only weakly pathogenic. However, it seems that when the A315S mutation occurs in cis to the S252L mutation, an entirely new, Apert-like syndactyly phenotype arises. Why might this be so?
The first point to note is that because the A315S mutation lies in the IIIc exon, the double mutation is only expressed in the FGFR2c form of the protein. Crucially, therefore, abnormality of this isoform, not FGFR2b, must lead to the syndactyly phenotype (we found no evidence of ectopic FGFR2b expression in fibroblasts cultured from II-9). A clue to how this could occur is provided by aligning the amino acid sequences of the IgIIIb and IgIIIc isoforms of FGFR1, FGFR2, and FGFR3 (the three FGFRs that exhibit alternative splicing) from a wide range of vertebrate species (Fig. 6). At the equivalent of the 315 position of IgIIIc in human FGFR2, all IgIIIc isoforms have an alanine and all IgIIIb isoforms have a serine. At only two other amino acid positions in these alternatively spliced exons is there such a clear-cut demarcation, suggesting that the 315 position is one of the critical determinants in conveying the distinct FGF-binding properties of these two alternatively spliced exons. According to the crystal structure of FGF2 bound to FGFR2c, the alanine is one of three residues forming a small hydrophobic core that stabilizes interactions with FGF2 [Plotnikov et al., 2000]. Further evidence for the importance of this residue in ligand selectivity comes from targeted mutagenesis experiments, which identified the H314-S315 dipeptide at the start of the IgIIIb domain as critical for conveying FGF7 binding to an FGFR1c construct [Wang et al., 1999].
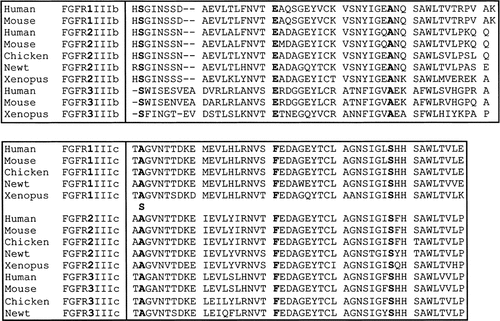
Amino acid sequence alignment of IgIIIb isoforms (above) and IgIIIc isoforms (below) of FGFR1, FGFR2, and FGFR3 from a range of vertebrate species. The A315S substitution is shown above the corresponding human FGFR2IIIc sequence. The three amino acids that differ consistently between the two isoforms are highlighted in bold type.
Our working hypothesis is that the A315S substitution conveys IgIIIb-like character to the IgIIIc protein isoform, at the same time weakening the intrinsic IgIIIc properties (Fig. 3E). It seems that the additional S252L substitution is necessary for the IIIb-like effect to be manifest, presumably by enhancing contacts specific to FGF7, FGF10, or a related ligand in a fashion analogous to, but distinct from, the S252W Apert mutation. Interestingly, in the purple urchin Stroxyglocentrotus purpuratus FGFR, the position equivalent to S252 is encoded by leucine, confirming that this amino acid can play a physiological role at this position in the linker region [Coulier et al., 1997]. We are currently undertaking biochemical analysis of this mutation to investigate its in vitro properties. Clearly, this family provides a precedent for FGFR2 mutations causing limb malformations without craniosynostosis.
CONCLUSIONS
A combination of surveys of the rich resource of human mutations, with biochemical and structural studies, has given us a good framework from which to account for the limb abnormalities associated with FGFR2 mutations. Figure 3 summarizes the major mechanisms that seem to be at work. We hope to have illustrated the complexity of the possible effects occurring at the biochemical and developmental levels. From a clinical perspective, an important conclusion is that disorders associated with FGFR2 mutations, especially Pfeiffer syndrome, are not single pathological entities but encompass a range of overlapping phenotypes. These reflect the precise pathological effects of the particular mutation on a complex combination of independent factors, including FGF affinity and selectivity, level of constitutive receptor activation, and pattern of spliceform expression.
Of course, not all mutations fit into this framework and these provide excellent starting points for further hypothesis testing. In this regard, future challenges will be to elucidate the mechanism of the A172F mutation in the eponymous Pfeiffer syndrome family, and the genetic basis of Philadelphia craniosynostosis. Although substantial progress has been made in understanding how mutations disturb function at a biochemical level, less is known about the phenotype at a developmental level. In other words, which of the many FGF-dependent processes in limb development are disrupted, and (equally intriguing) how do the other processes remain intact? To address these questions, mice with Fgfr2 mutations that recapitulate the syndactyly phenotype are needed.
The contribution of FGFR1 mutation in limb abnormalities is practically uncharted territory. Not only is the single definite mutation of human FGFR1 associated with limb malformation, but two different engineered mutations of the mouse orthologue Fgfr1 also cause limb malformations [Partanen et al., 1998; Lalioti et al., 2001]. We know that FGFR1 is expressed in the limb mesenchyme at the right time [Deng et al., 1997; Xu et al., 1999]. We are currently undertaking a genomic screen of FGFR1 in patients with heterogeneous limb malformations to determine whether additional mutations of this gene can be identified; to date, however, no clearly pathogenic mutation has been found in 91 independent cases (S.-h. Kan and A.O.M. Wilkie, unpublished data).
Finally, little is known about the contribution of FGF mutations to human limb malformations. Perhaps we should anticipate a lesser impact, for reasons stated in the section on mutation spectrum. Our own group has conducted a screen for FGF10 mutations in a cohort of 24 patients with heterogeneous limb malformations, with negative results (data not shown). However, the exact physiological role of many FGFs in limb development remains to be delineated; until that time, it is difficult to be certain that mutation searches have been focussed on the most appropriate combinations of FGFs and candidate phenotypes.
Acknowledgements
We thank Max Muenke for generously contributing the sample from the patient with Philadelphia craniosynostosis to this study, John Heath, Yvonne Jones, and Gillian Morriss-Kay for fruitful discussions, and Stephen Robertson for comments on the article. Financial support for this work was provided by the Wellcome Trust (to A.O.M.W.), Medical Research Council, UK. (to S.J.P.), and the Ministry of Education in Taiwan and Overseas Research Students Awards Scheme (to S.-h.K.).




