Kallmann syndrome in a patient with congenital spherocytosis and an interstitial 8p11.2 deletion
Abstract
We describe the hitherto smallest interstitial 8p11.2 deletion in a patient with congenital spherocytosis, dysmorphic features, and growth delay in association with hypogonadotropic hypogonadism and anosmia. The latter features are characteristic for Kallmann syndrome. In contrast to the previously reported patients with 8p deletions, the present patient showed normal intelligence. Congenital spherocytosis is one of the most common hereditary hemolytic anemias. One of the three loci for congenital spherocytosis was assigned to chromosome 8p (located between 8p11.1 and 8p21) and mutations in or loss of the ankyrin-1 gene (ANK1) were identified. Molecular analysis confirmed the de novo loss of ANK1 in our patient. Kallmann syndrome, which is characterized by hypogonadotropic hypogonadism and anosmia, can be X-linked, autosomal dominant, or autosomal recessive. So far only the X-linked KAL1 gene has been identified. The present finding suggests an autosomal locus for Kallmann syndrome at 8p11.2. The simultaneous occurrence of congenital spherocytosis, Kallmann syndrome phenotype, dysmorphic features, and growth delay in this patient points to a new contiguous gene syndrome. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Deletions of the proximal part of 8p (8p11.1 to 8p21) are typically associated with congenital spherocytosis, which is one of the most common hereditary hemolytic anemias (1/5,000 in the general northern European population). The description of seven patients with 8p deletions and congenital spherocytosis allowed identification of a locus for congenital spherocytosis on 8p11.2. Since then the ankyrin-1 gene (ANK1) was located at 8p11.2 and mutations in ANK1 were identified in several congenital spherocytosis patients [Okamoto et al., 1995; Eber et al., 1996].
We describe the hitherto smallest cytogenetically detected 8p11.2 deletion in a patient with congenital spherocytosis, mild dysmorphic features, and growth retardation in association with hypogonadotropic hypogonadism and anosmia, which are the typical features of Kallmann syndrome. So far, the only genes linked to Kallmann syndrome were the X-linked KAL1 gene on Xp22.3 [Ballabio et al., 1989] and the gonadotropin releasing hormone receptor gene (GNRHR) on 4q21 involved in autosomal recessive Kallmann syndrome [Layman et al., 1998; Kottler et al., 1999; de Roux et al., 1999]. Sixty-six percent of the Kallmann syndrome patients are sporadic. Of the familial cases 11% are X-linked, 25% show an autosomal recessive inheritance, and the majority (64%), show an autosomal dominant inheritance [Seminara et al., 1998]. Although mutations have been found both in KAL1 and in GNRHR, the genetic defect in patients with Kallmann syndrome remains unknown in the majority of cases. So far, Kallmann syndrome has not been reported in association with autosomal microdeletions.
The spectrum of the phenotypic association observed in the present patient suggests that an autosomal locus for Kallmann syndrome resides in close proximity to the ANK1 gene and points to a new and previously unrecognized microdeletion syndrome.
CLINICAL REPORT
The index patient is the second son born to healthy, non-consanguineous parents. Pregnancy and delivery at 41 weeks of gestation were normal, but no information is available on birthweight length or head circumference. At birth, phototherapy was necessary because of a rapidly progressive jaundice. The boy was admitted to the children's hospital at the age of one month for treatment of a severe anemia. Osmotic fragility studies disclosed the presence of spherocytes, suggesting the diagnosis of congenital spherocytosis. During childhood recurrent episodes of asthenia, jaundice, and abdominal pains occurred, for which a therapeutic splenectomy was performed at the age of 14 years. There was no regular follow-up before the age of 14.5 years. At that time, the boy was examined at the pediatric department because of progressive growth retardation, with a progressive deflection of the growth chart toward the third percentile. The height was 152 cm (±P3). Tanner's staging showed prepubertal signs. Subsequent endocrinological investigations revealed a growth hormone deficiency (less than 10 ng/ml). Treatment with growth hormone was initiated at the age of 17 years (height: 155 cm, P3 = 159 cm; 39.4 kg, P3 = 49 kg), but height remained below the third centile and pubertal signs remained absent. At age 20 years, length was 171 cm (P25–P50) and weight was 50 kg (±P3). Because of hypogonadism and delayed masculinization, further endocrinological investigations were performed showing a hypogonadotropic hypogonadism (LH < 0.7 mU/ml, normal values 1–12 mU/ml; FSH < 0.1 mU/ml, normal values 1–12 mU/ml). However, at that time a sperm analysis was not done. The cause of hypogonadotropic hypogonadism remained unknown. A treatment with testosterone was started. Meanwhile, the patient got married and the couple planned to have children. At age 23 years, the patient was referred to the Center for Medical Genetics because of infertility. Clinical examination confirmed the hypogonadism with a testicular volume of 6 ml. In addition minor facial anomalies were noted with bilateral crumpled ears and micrognathia. Hearing and intelligence were normal. Further history taking revealed a problem of anosmia that the patient had not spontaneously mentioned on previous occasions. The patient mentioned that he was unable to smell any perfume, food odors, or any other odors since childhood. No abnormalities were noted on rhinoscopy. The patient declined radiological examinations for visualization of the olfactory bulb. Family history was normal with respect to the presence of spherocytosis, anosmia, or endocrinological problems. A sperm analysis revealed complete azoospermia. The combination of hypogonadotropic hypogonadism, azoospermia, and anosmia suggested Kallmann syndrome (OMIM: X-linked: 308700, autosomal dominant: 147950, autosomal recessive: 244200). A cytogenetic analysis was performed, which revealed a small deletion of the short arm of chromosome 8: 46,XY,del(8)(p11.2).
Cytogenetic Investigations
Analysis of G-banded prometaphase chromosomes from short-term lymphocyte cultures of the proband showed a small interstitial deletion on the short arm of chromosome 8: 46,XY,del(8)(p11.2) (Fig. 1a, b). Both parents had normal karyotypes. Fluorescence in situ hybridization (FISH) with the locus specific LSI-KAL probe (Vysis, Downers Grove, IL) for the KAL1 gene showed a normal fluorescent signal on Xp22.3, excluding submicroscopic deletion of KAL1 in our patient. FISH with YAC probe 774C1 containing the GNRH1 gene [Adelaide et al., 1998] showed a normal fluorescent signal on both chromosomes 8 which excluded this gene from the deleted region.
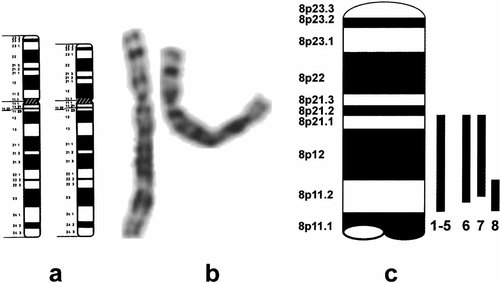
Ideograms (a) and representative G-banded partial karyotypes (b) from the patient carrying the deletion (left, normal chromosome; right, abnormal chromosome). c: Schematic presentation of the 8p deletions described in literature. 1 and 2: Cohen et al. [1991]; 3 and 4: Chilcote et al. [1987]; 5: Lux et al. [1990]; 6: Kitatani et al. [1988]; 7: Okamoto et al. [1995]; 8: this patient.
Molecular Investigations
DNA was extracted from peripheral blood of the proband and from his parents using a QiAamp DNA blood kit (Qiagen GmbH, Hilden, Germany). The following microsatellite markers were analyzed as described previously [Messiaen et al., 1996]: D8S560; D8S1820; D8S255; D8S268; D8S531 (Fig. 2a). Marker analysis of D8S255 and D8S268 (Ankyrin, ANK1) indicated deletion of the paternal alleles (Fig. 2b). D8S531 residing 1 cM proximal from ANK1 was not involved in the microdeletion. Since the gonadotropin releasing hormone 1 gene product (GNRH1) may be a candidate gene for Kallmann syndrome, we investigated markers distal (D8S560) and proximal (D8S1820) to this gene. These markers reside respectively 20 and 10 cM distal from the ANK1 gene. Both markers D8S560 and D8S1820 flanking GNRH1 were not deleted (Fig. 2a).
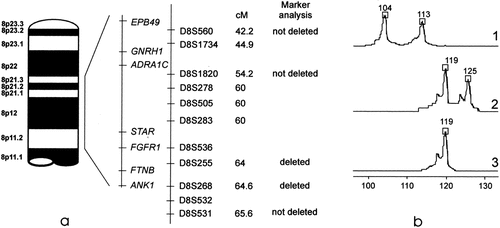
a: Schematic presentation of candidate genes and polymorphic markers in the 8p11.2 region. Genetic distances of the markers indicated in centimorgans (cM) were derived from GeneMap 99 (www.ncbi.nlm.nih.gov) and Marshfield genetic database (www.marshmed.org). Acronyms for the respective genes: ANK1, Ankyrin 1; ADRA1C, adrenergic alpha-1C-receptor; EPB49, erythrocyte membrane protein; FGFR1, fibroblast growth factor receptor 1; FTNB, fertilin beta; GNRH, gonadotropin releasing hormone; STAR, steroidogenic acute regulatory protein. b: Representative analysis of marker D8S255; 1, paternal alleles; 2, maternal alleles; 3, alleles from the index patient. Figures indicate respective allelic lengths in base pairs.
DISCUSSION
We present a patient with an 8p11.2 microdeletion, congenital spherocytosis, subtle facial anomalies, a history of growth delay, and normal intelligence in association with Kallmann syndrome phenotype.
Congenital spherocytosis is caused by mutations in or loss of the ankyrin-1 gene (ANK1) which is implicated in linking integral membrane proteins to the cytoskeleton [Lux et al., 1990; Eber et al., 1996]. Molecular analysis confirmed the de novo loss of the ANK1 gene in the present case. The patients with 8p11.2-p21 deletions described in literature [Okamoto et al., 1995] have severe mental retardation, short stature, primary failure of sexual development associated with low LH and FSH levels resulting in hypogonadotropic hypogonadism, and dysmorphic features including micrognathia (7/7), microcephaly (5/7), preauricular pits (3/7), high arched palate (2/7), abnormal ears (2/7), and rib anomalies (2/7). Anosmia was not investigated in these patients. Our proband had only minor dysmorphic signs including micrognathia and crumpled ears. Although in the previously described patients no molecular delineation of the breakpoints was performed, the present case probably represents the smallest hitherto reported interstitial 8p deletion. The small size of the deletion in this case most likely explains the normal intelligence in this patient in contrast to the other published patients with 8p deletions and congenital spherocytosis (Fig. 1c).
Although mutations have been found in the X-linked KAL1-gene, the majority of familial cases with Kallmann syndrome remain unexplained [Seminara et al., 1998]. Since hypogonadotropic hypogonadism results from the failure of normal pulsatile GNRH1 secretion, GNRH1 may be a candidate gene for Kallmann syndrome [Seminara et al., 1998]. Deletion of the mouse homolog of GNRH1 was found in the hypogonadal mouse in which the phenotype was completely reversible by insertion of the normal gene [Mason et al., 1986a, b]. However, no deletions or mutations of GNRH1 have been found in human studies so far [Seminara et al., 1998]. GNRH1 has been mapped on 8p11.2-p21 [Yang-Feng et al., 1986]. Molecular analysis and FISH showed the presence of GNRH1 in our patient, thus excluding deletion of GNRH1 as the cause of Kallmann syndrome (data not shown). Based upon the clinical and genetic observations in the present patient we propose the existence of a new contiguous gene syndrome caused by an 8p deletion and characterized by congenital spherocytosis and a Kallmann syndrome phenotype. Mutation analysis will be performed in our patient for GNRH1 since we could only exclude large deletions with FISH. One of the most promising candidate genes residing in the deleted region is FTNB, which results in infertility in a mouse model [Cho et al., 1998]. Further studies will aim at a refined molecular delineation of the 8p deletion in Kallmann syndrome-congenital spherocytosis patients and a renewed clinical investigation in 8p deletion patients in order to define the deleted genes of interest.




