Infections in patients with chronic lymphocytic leukemia treated with time limited targeted drug combinations
In chronic lymphocytic leukemia (CLL) immune dysregulation heightens infection susceptibility.1 Targeted therapies enhance treatment outcomes but pose infection-related concerns. In this context, we evaluated infections in a well described population of clinical phase II/III patients of the German CLL Study Group (Supplementary Statistical Methods). The trials were approved and registered at clinicaltrials.gov. Patients provided written consent as per the Declaration of Helsinki.
Treatment-emergent infections, occurring up to 28 days post the final exposure to a targeted drug, were analyzed in a cohort of 1184 patients (venetoclax: 723, BTKi: 128, BTKi plus venetoclax: 297, idelalisib: 36; Table S1) with varying median treatment durations (venetoclax: 11 months, BTKi: 20.4 months, BTKi plus venetoclax: 11.9 months, idelalisib: 13.4 months). All patients had received an anti-CD20 antibody (obinutuzumab: 888, rituximab: 231, ofatumumab: 65), and 182 patients had received bendamustine de-bulking prior start of targeted treatment.
At a median reporting duration of 14.7 months (range 0.2–34.3), 65.1% of patients experienced a total of 1812 treatment-emergent infections, with a median of two infections per patient (range 1–14) and a median time to first infection of 6.1 months (range 0–34.3). The majority of treatment-emergent infections were of lower CTC grade 1–2 (85.5%) and 52.6% occurred in the first 6 months of treatment (Tables S2–S3). Most common infections were nasopharyngitis (16%), upper respiratory tract infection (7.5%), pneumonia (7.1%), and urinary tract infection (7%).
Standard values were selected for the specific cutoffs for serum IgA (≥0.7 g/L), IgG (≥7 g/L), and IgM (≥0.4 g/L). IgA deficiency was associated with an increased risk for developing treatment-emergent infections (p = .029; odds ratio [OR] 1.312; 95% confidence interval [CI] 1.029–1.672), whereas IgG or IgM deficiency was not prognostic. An increased comorbidity index rating scale (CIRS) score of >6 was associated with a lower risk of infections (p = .003; OR 0.633; 95% CI 0.47–0.853).
When comparing lines of treatment, 73.4% of 143 relapsed or refractory patients had an infection versus 64% of 1041 treatment-naïve patients (p = .027; OR 1.556; 95% CI 1.051–2.303).
When testing against venetoclax treated patients, more patients had an infection that were BTKi treated (p < .001; OR 3.093; 95% CI 1.923–4.974), BTKi plus venetoclax treated (p = .004; OR 1.534; 95% CI 1.15–2.045), or idelalisib treated (p = 0.016; OR 2.807; 95% CI 1.213–6.493).
When testing against obinutuzumab, the infection risk was increased in patients receiving ofatumumab (p < .001; OR 5.3; 95% CI 2.263–12.414), but not in patients receiving rituximab (p = .066; OR 0.758; 95% CI 0.564–1.019).
Bendamustine de-bulking prior start of targeted treatment was associated with an increased infection risk (p = 0.006; OR 1.653; 95% CI 1.158–2.36).
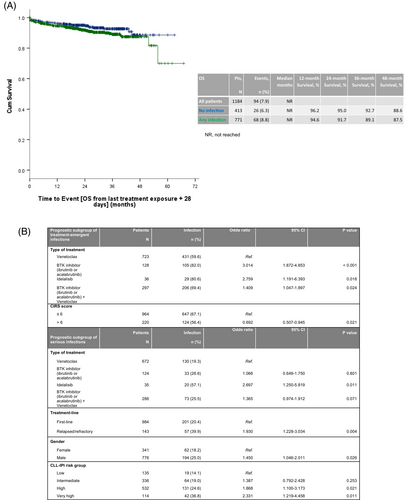
Other pretreatment characteristics such as age, gender, Binet stage, creatinine clearance, or CLL international prognostic index risk group had no impact on the risk of treatment-emergent infections (Table S5). As type of treatment, line of treatment, CIRS score, IgA deficiency, and bendamustine de-bulking were significantly associated with infections in univariable analyses with a significance level of 5%, they were considered for multivariable modeling (type of administered anti-CD20 monoclonal antibody was excluded due to correlations with type of treatment). In multivariable testing CIRS score and type of treatment were identified as independent factors for the development of treatment-emergent infections (Figure 1B).
A total of 366 patients developed severe neutropenia or leukopenia of CTC grade 3–5 during the course of treatment. In these patients, 878 treatment-emergent infections were documented. In comparison, 934 infections were reported in 818 patients that did not develop severe neutropenia or leukopenia. Severe infections of higher CTC-grade (3–5) occurred in 14.7% of cases with severe neutropenia/leukopenia and 14.3% in those without (Table S4).
A total of 1035 treatment-emergent infections were classified by causative pathogens (bacterial: 546, viral: 405, fungal: 84), including nine fatal cases (bacterial: three, viral: four, fungal: four—one case of sepsis was classified as bacterial, viral, and fungal in one patient).
Compared to patients treated with venetoclax, more patients that received BTKi plus venetoclax (p < .001; OR 3.113; 95% CI 1.830–5.296) or idelalisib (p < 0.001; OR 8.593; 95% CI 3.685–20.037) suffered treatment-related fungal infections. No further prognosticators for the development of fungal infections were identified, likely explained by its low overall prevalence of 6.5%.
The estimated 24-month survival rate after last treatment exposure of a targeted drug was 91.7% with 68 (8.8%) documented deaths in the group of 771 patients who had experienced at least one treatment-emergent infection. This was compared to an estimated 24-month survival rate of 95% in the group of 413 patients who remained infection-free, with 26 (6.3%) documented deaths. The hazard ratio for survival between these two groups was 1.481, with a 95% CI ranging from 0.942 to 2.327 (Figure 1A).
When assessing survival from last treatment exposure separately for 1041 first-line and for 143 relapsed or refractory patients (Figures S1–S2), infections showed no impact on survival in either subgroup.
Upon analyzing survival according to type of pathogen, the estimated 24-month survival rates post-last treatment exposure were: 84.6% for bacterial infections, 97% for viral infections, 83.3% for fungal infections, and 92.9% for cases involving multiple pathogens (Figure S3).
Serious infections were to be reported regardless of the time elapsed from the last administration of targeted drug and the median reporting duration was 40.1 months (range 0.9–76.3). A total of 417 serious infections were reported in 23.1% of patients with a median of one serious infection (range 1–6), including 217 first-line and 57 relapsed or refractory patients. Majority of serious infections occurred within the first 6 months (35.5%), were in 88.7% of cases of CTC grade 3–5, including 32 lethal infections in 31 patients. The most common serious infections were pneumonia (27.1%), sepsis (8.4%), urinary tract infection (7%), SARS-CoV-2 infection (6%), and influenza (3.6%).
In comparison with venetoclax-treated patients, the risk to develop serious infections was significantly higher in idelalisib-treated patients (p < .001, OR 4.983; 95% CI 2.519–9.856). With a significance level of 5%, univariable analysis identified line of treatment, type of administered anti-CD20 monoclonal antibody, age (≤65 years vs. >65 years), gender, and CLL-IPI risk group as further variables associated with an increased risk of serious infections, whereas bendamustine de-bulking was not identified as a prognostic factor (Table S6). Multivariable testing identified kinase-inhibitor based treatment, advanced treatment line, male gender, and high or very high CLL-IPI risk group as independent factors (Figure 1B).
Overall infection rates in first-line phase three studies of targeted drug combinations2 match those for chemoimmunotherapy, affecting approximately 60%–75% of patients. However, the severity of infections varies depending on the treatment regimen.
In our dataset of 1184 patients receiving targeted drugs, treatment-emergent infections occurred in 65.1% patients, with more than 15% of cases being of higher CTC grade. These infections did not impact overall survival, with over 50% of all treatment-emergent infections occurring within the first 6 months of treatment and predominantly affecting the respiratory tract and classified as bacterial or viral. Fungal infections were rare with an overall prevalence of 6.5% including four lethal cases.
Serious infections were reported in 23.1% of patients, including cases of pneumonia, sepsis, urinary tract, and SARS-CoV-2 infection.
While previous studies highlighted infections as a major concern in CLL,3 our analysis suggests that while treatment-emergent infection remains a challenge with targeted therapies, they no longer seem to impact overall survival and our results suggest an improvement of the CLL-associated immune dysfunction. In line with this observation, previous investigations demonstrated a meaningful immune restoration for several targeted drugs.4, 5
Within univariate analyses, hypogammaglobulinemia of IgG or IgM was no prognosticator for the risk of treatment-emergent infection, as opposed to IgA-deficiency. Historically, decreased immunoglobulin levels correlated with severe infections in chemotherapy-treated patients, leading to intravenous immune globulin approval for infection prophylaxis in CLL.6 Concluding from our dataset the overall level of Ig deficiency did not impact the infection rate. However, since no data on IVIG administration were collected, we cannot exclude that potential immune globulin replacement may have resulted here in a beneficial effect.
Multivariable analysis suggested BTKi-based treatment and lower comorbidity burden as risk factors for treatment-emergent infections. Serious infections appear to be influenced by kinase-inhibitor treatment, advanced treatment line, male gender, and higher CLL-IPI risk group. These results should be interpreted with caution due to the longer treatment duration with BTKi and higher proportion of relapsed or refractory patients in the kinase inhibitor treatment group.
When evaluating comorbidity in the context of treatment-emergent infections, we have to point out that among 220 patients with a CIRS > 6, only 13.6% were treated with BTKi-based combinations and >80% were treated with venetoclax. Compared to this, 41% of 964 patients with a CIRS ≤ 6 were treated with a BTKi-based combination. The median treatment duration did not really differ between patients with and without relevant comorbidity with 10.4 and 11.1 months, respectively. Considering this, type of targeted treatment may be a possible explanation for risk of infection. Additionally, patients with higher comorbidity may have been managed more cautiously due to their preexisting health conditions, potentially mitigating the risk of treatment-related infections.
In summary, patients with CLL treated with targeted drugs still are at increased infection risk, but infections do not seem to be indicative of overall survival.
AUTHOR CONTRIBUTIONS
PL was responsible for the conception and design of the analysis. AG and SR performed the analysis. PL and BE wrote the first draft of the manuscript. All authors contributed data for the analysis, revised the manuscript, and approved the final version.
CONFLICT OF INTEREST STATEMENT
PL: Honoraria and personal fees from AbbVie, AstraZeneca, BeiGene, Janssen. Research funding from Janssen. AG: None. SR: Honoraria from AstraZeneca and MSD. PC: Research funding by Acerta, AstraZeneca, BeiGene, F. Hoffmann-La Roche, Gilead, Janssen-Cilag, and Novartis; honoraria by AbbVie, AstraZeneca, BeiGene, BMS, F. Hoffmann-La Roche and Janssen-Cilag, advisory boards by Acerta, AstraZeneca, BeiGene, Janssen-Cilag, and Novartis; travel support by AbbVie, AstraZeneca, BeiGene, F. Hoffmann-La Roche, Gilead, Janssen-Cilag, and Novo Nordisk. JvT: Honoraria AbbVie, AstraZeneca, Beigene, Janssen, Lilly, Roche. Advisory Boards AbbVie, Amgen, AstraZeneca, Beigene. Travel support AbbVie, AstraZeneca, Beigene, Janssen, Lilly, Roche. OAS: Honoraria from AbbVie, Adaptive, Ascentage, AstraZeneca, BeiGene, Eli Lilly, Genmab, Gilead, Janssen, Roche. Research funding from AbbVie, BeiGene, Janssen, Roche. AMF: Grants and consulting fee from AstraZeneca. Research support by AstraZeneca and Celgene. MF: Conflict of Interest: research grants by AbbVie, AstraZeneca, BeiGene, Janssen, Roche, honoraria by AbbVie. AK: Research grants from AbbVie, Astra Zeneca, BMS, Janssen, and Roche. Advisory board for AbbVie, Astra Zeneca, BMS, Janssen, LAVA, and Roche Genentech. EVS: Teaching activities for Novartis and Janssen. CUN: Research grants by AstraZeneca, Gilead. Honoraria by AbbVie, AstraZeneca, BMS, Kite/Gilead. CCB: Consulting fee by Janssen; Payment or honoraria for lectures, presentations: Octapharma. Support for attending meetings and/or travel: Abbvie, Octapharma. Participation on a Data Safety Monitoring Board or Advisory Board: Janssen, Astra Zeneca, Beigene. ET: Grants from Roche. Grants, personal fees, and nonfinancial support from Abbvie. Personal fees and nonfinancial support from Janssen. CS: Honoraria from AstraZeneca and AbbVie. Advisory Board for Janssen-Cilag. SS: Advisory board honoraria (AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis), Research support (AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis), Travel support (AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis), Speaker fees (AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis). KF: Honoraria from AbbVie and Roche. Advisory Board for AstraZeneca. MH: Personal fees and other (AbbVie, Celgene, Gilead Sciences, Janssen, Pharmacyclics, Roche). Honoraria (speaker's bureau and/or advisory board) by Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca. Research support by Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca. BE: Grants from Janssen-Cilag, Roche, Abbvie, Astra Zeneca, BeiGene. Consulting fees from Janssen-Cilag, MSD, Lilly, Abbvie, Astra Zeneca, BeiGene. Honoraria from Janssen-Cilag, MSD, Lilly, Abbvie, Astra Zeneca, BeiGene, Roche.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.