Iron restriction in sickle cell disease: When less is more
Abstract
Primum non nocere! Can iron deficiency, an abnormality that causes anemia, benefit people with sickle cell disease (SCD) who already have an anemia? The published literature we review appears to answer this question in the affirmative: basic science considerations, animal model experiments, and noncontrolled clinical observations all suggest a therapeutic potential of iron restriction in SCD. This is because SCD's clinical manifestations are ultimately attributable to the polymerization of hemoglobin S (HbS), a process strongly influenced by intracellular HbS concentration. Even small decrements in HbS concentration greatly reduce polymerization, and iron deficiency lowers erythrocyte hemoglobin concentration. Thus, iron deficiency could improve SCD by changing its clinical features to those of a more benign anemia (i.e., a condition with fewer or no vaso-occlusive events). We propose that well-designed clinical studies be implemented to definitively determine whether iron restriction is a safe and effective option in SCD. These investigations are particularly timely now that pharmacologic agents are being developed, which may directly reduce red cell hemoglobin concentrations without the need for phlebotomies to deplete total body iron.
1 INTRODUCTION
Sickle cell disease (SCD), a group of inherited disorders characterized by mutations in both alleles encoding the hemoglobin subunit β (HBB), is estimated to affect approximately 5.7 million individuals globally.1, 2 All forms of SCD are characterized by at least one HBB allele being βS. The presence of βS homozygosity is diagnostic for sickle cell anemia (SCA), the most common form of SCD, and a genotype of βS/βC allele results in hemoglobin SC disease (HbSC). Finally, HbSβ-thalassemia results from a βS allele combined with either β0 (a null HBB allele) or β+ (a hypomorphic HBB allele) thalassemia.1 Both HbSC and HbSβ+ generally result in clinical disease that is milder than SCA, particularly in childhood and young adulthood.3 Whereas patients with SCA commonly have hematocrits of 20%–30%, the hematocrits of patients with HbSC and HbSβ+ are typically 30%–36% and 28%–34%, respectively.4-6
Because individuals who have sickle cell trait (i.e., AS heterozygotes) exhibit partial protection from malaria, SCD prevalence has historically been highest in regions where malaria is endemic.6, 7 Today, approximately 300 000 infants affected by SCD are born annually worldwide, and an estimated 100 000 individuals in the United States have SCD.8-10 Although new pharmacologic options have been developed and a few of them approved, the burden of SCD remains high, particularly in regions where access to therapy is limited. For example, in sub-Saharan Africa, more than 50% of newborns with SCD do not survive 5 years.11 In regions with better access to high-quality healthcare, including medications and blood transfusions, such as the United States, life expectancy for individuals with SCD still remains 20–30 years shorter than for those without the disease.12-14
The clinical manifestations of SCD are attributable to biophysical changes in red blood cells (RBCs) that result from polymerization of hemoglobin S (HbS). RBCs with HbS polymers are rigid, less deformable, and assume an abnormal holly-leaf or sickled shape. This impairs their intravascular circulation and survival, as well as their ability to deliver oxygen to tissues (vaso-occlusion). Polymerization also damages the RBC membrane, resulting in abnormal RBC adhesion to the endothelium and further impairment of RBC circulation.1, 15 HbS polymerization may be increased by many variables, such as decreased partial pressure of oxygen, reduced temperature, relative dehydration, reduced pH, and increased concentrations of 2,3-diphosphoglycerate (2,3-DPG).1, 16 Importantly, free heme and free iron within the erythrocyte cytosol have also been associated with increased HbS polymerization.17, 18 The strongest variable affecting HbS polymerization in hemoglobin solutions, however, is the concentration of HbS. This is because HbS polymerization is not an instantaneous process; it occurs in stages of nucleation, growth, and secondary nucleation.1, 19 Rapid polymer growth cannot occur until 10 deoxy-HbS tetramers assemble to form a nucleating hemoglobin aggregate (nucleus).20 This delay time, or latency period, between nucleus formation and polymerization is greatly affected by the HbS concentration: halving the concentration of HbS increases the delay time by a factor of 3 million, suggesting that even small decrements in intracellular HbS concentrations can markedly reduce polymerization and improve the clinical severity of SCD.21-23
Recognizing that iron deficiency reduces RBC indices such as mean corpuscular hemoglobin concentration (MCHC) in healthy individuals and those with sickle trait,24 in 1973 Lincoln et al. hypothesized that induction of iron deficiency in people with SCD could be therapeutic.25 Specifically, they wrote that “The therapeutic implications are clear. If the mean corpuscular haemoglobin concentrations can be manipulated on the down side without serious detriment to the patient, then the sickling process may be controllable and crises averted in persons with [homozygous] SS disease” and that “The net effect [of a reduction in haemoglobin concentration] would be to replace an anaemia characterised by excessive red-cell destruction by a less detrimental microcytic hypochromic anaemia.” In the intervening 50 years, this iron restriction hypothesis as potential therapy has been supported by observational studies, case reports, and especially by well-designed experiments in animal models. The concept that low MCHC improves SCA is also supported by observations in patients with coexistent α-thalassemia. These patients' RBCs have an α-thalassemia–related MCHC reduction that, although of lower magnitude than that seen with iron deficiency, is still associated with decreased hemolysis and a higher hemoglobin level.26, 27 At a recent meeting of international experts in the field, we decided to undertake this updated literature review to assess the evidence for Lincoln's hypothesis. We also examine in-development pharmacologic therapies focusing on the modulation of iron homeostasis, decreasing intra-erythrocyte iron, and achieving MCHC reduction without the need for phlebotomies to deplete body iron.
2 IRON METABOLISM AND HOMEOSTASIS IN PATIENTS WITH SCD
In healthy populations, iron status is generally examined by assessing a complete blood count with RBC indices and morphology, serum ferritin levels, and transferrin saturation (TSAT) levels. When necessary, more advanced laboratory markers (e.g., erythrocyte zinc protoporphyrin, hepcidin, soluble transferrin receptors, reticulocyte hemoglobin concentration, non–transferrin-bound iron [NTBI]), magnetic resonance imaging, and bone marrow biopsies can be performed.28 SCD is generally characterized by normal iron distribution and iron stores or relative iron deficiency.29, 30 With the possible exception of iron accumulation in the kidney related to chronic intravascular hemolysis, nontransfused patients with SCD do not exhibit clinical, laboratory, or radiologic evidence of iron overload.23, 31
Interpretation of serum ferritin levels as a marker of iron status in SCD is complicated by the role of ferritin as an acute phase reactant and the high prevalence of vascular and systemic inflammation, triggered by recurrent vaso-occlusion.1, 15, 32 Nevertheless, a low ferritin level is specific—even in the setting of SCD—for decreased body iron. Hepcidin and erythroferrone are key regulators of iron homeostasis, but data examining the impact of SCD on these hormones suggest a complex relationship. Whereas ineffective erythropoiesis and chronic hemolytic anemia would be expected to reduce hepcidin concentrations, inflammation and increased serum iron concentrations would be expected to induce hepcidin expression.33, 34 Fluctuating inflammation in SCD may help explain the seemingly conflicting data about hepcidin and ferritin levels in SCD. For instance, Ezeh et al. found similar hepcidin levels among healthy adults and adults with SCA, including those patients with increased rates of clinical complications.35 In contrast to healthy adult controls, however, ferritin levels were generally increased in SCA; this was attributed to a combination of inflammation and transfusion therapy. In a separate cohort of adults with SCD, Kroot et al. found that the hepcidin-25/ferritin ratio—a measure of appropriateness of hepatocyte-produced hepcidin for the iron burden—was significantly lower in people with SCD (N = 16) relative to race-matched patients with HbAS.36 In a larger cohort, Omena et al. found that serum hepcidin concentrations in SCA were lower than those observed in the control group of patients with a hemoglobin AA genotype.33 In SCD, an upregulation of erythroferrone appears to contribute to reduced hepcidin concentrations, but the correlation appears weaker than that observed in patients with β-thalassemia.37, 38 Omena et al. found that the highest hepcidin levels were observed in patients with SCD with possible iron overload, as indicated by serum ferritin levels ≥1000 ng/mL.33
At baseline, people with SCD do not appear to be at risk for iron overload. Recurrent blood transfusions clearly result in iron overload (although less than that observed in patients with other chronic hemolytic disorders such as β-thalassemia). When iron overload does occur in patients with SCD who are receiving transfusion therapy, it is less likely to involve the endocrine glands and heart, remaining predominantly in the liver and spleen.39 Despite a lower overall risk of iron overload and its complications, patients with SCD receiving chronic transfusions should have liver iron levels assessed every 1–2 years. Unfortunately, data suggest that current screening rates are suboptimal, with only 32% and 41% of children and adults, respectively, receiving appropriate assessments.40
Low RBC deformability41 and decreased intravascular survival42 can be features of severe iron deficiency. Hence iron restriction could theoretically add to hemolysis in patients with SCD. However, RBC survival tests in two iron-deficient patients with SCD showed their RBC life span to be longer than with iron repletion.43, 44 This paradox could be explained if any iron deficiency–related reduction in RBC survival were overcompensated by a more robust decrease in sickle-related hemolysis.
An important consideration is the possibility that iron deficiency could worsen the anemia in patients with SCD by adding a low RBC production state to their ongoing increased RBC destruction. Patients with SCA and severe inhibition of marrow erythropoiesis from parvovirus B19 infection, for example, develop a life-threatening anemia with a mean hemoglobin level of 3.9 g/dL.45 In patients with SCD who are phlebotomized to induce iron deficiency, however, the decrease in hemoglobin level is not nearly as severe, at roughly 18% lower than baseline.46 Furthermore, some studies in sickle mice with iron deficiency have shown that the hemoglobin level either increases or remains unchanged.47-50 We propose that the main reason for the difference in hemoglobin reductions seen in these two types of hypoproliferative state is that iron deficiency–related decreases in RBC production are balanced by much greater decreases in hemolysis (from low MCHC), so the net effect is a milder reduction in hemoglobin level.
3 IRON DEFICIENCY AND IRON TREATMENT IN PATIENTS WITH SCD—OBSERVATIONAL DATA
To critically evaluate published evidence on the relationship between low iron status and disease severity in SCD, the authors performed a literature search for records within the MEDLINE (PubMed, US National Library of Medicine, National Institutes of Health) database on the terms iron and sickle cell through March 31, 2023. The resultant titles (~1500) and abstracts were reviewed, and full-text articles were retrieved for relevant records. The authors also identified relevant posters presented at medical congresses. A formal systematic review of these sources was not performed, and no formal grading of the quality of evidence was performed.
In 1982, Haddy and Castro described four cases of SCD with overt iron deficiency resulting from blood loss (aspirin intake, menorrhagia, ulcerative colitis, and endometrial hyperplasia).51 During the period of iron deficiency, the patients experienced a total of two pain crises over 4.2 patient years of observation. In all four cases, the abnormality underlying blood loss was corrected, and three patients received iron therapy. During the period of iron repletion, the four patients experienced 17 pain crises over 2.6 patient years of follow-up. Additional studies conducted for one patient demonstrated that RBC survival was reduced by ~66% following treatment, and that the proportion of irreversibly sickled cells increased from 0.3% during iron deficiency to 7.7% after iron treatment.43 In 1983, Rao et al. described a patient with SCD with severe iron deficiency.52 While iron deficient, the patient experienced no pain crises and exhibited infrequent (0.02%–0.23%) sickled RBCs on a peripheral blood smear. Following 6 weeks of oral iron therapy and a partial exchange transfusion, a marked increase in sickled cells (2.2%) was observed. In 2009, Castro et al. reported the case of a woman with SCA and longstanding iron deficiency secondary to gynecologic pathology.53 Treatment with oral iron supplementation was followed by increases in hemoglobin levels, reticulocyte counts, mean corpuscular hemoglobin (MCH), and MCHC, but the patient also demonstrated evidence of markedly increased hemolysis. Specifically, she experienced a 370% increase in lactate dehydrogenase (LDH) levels, a 66% increase in bilirubin concentration, and a nearly 10% decrease in RBC count. These observational data, summarized in Table S1, suggest that iron deficiency in SCA confers beneficial effects, whereas iron repletion causes hematologic changes associated with more severe clinical disease.
The impact of iron status on SCD has also been examined in a pair of cross-sectional studies. In 2012, Cox et al. examined the relationship between TSAT and nocturnal hemoglobin oxygen desaturation in 32 nontransfused children with SCA (HbSS).54 Increased TSAT levels were associated with more frequent and more severe nocturnal hemoglobin oxygen desaturations, a marker of increased rates of painful crisis and vascular dysfunction.54 More recently, Parrow et al. presented (in poster form) an analysis of laboratory parameters from a database of nearly 800 patients with SCD (all genotypes).55 Both MCHC and TSAT were inversely correlated with RBC counts, suggesting that reduced iron availability improved hematologic parameters in these patients. No clinical event data were included in the analysis. These cross-sectional studies further support the hypothesis that patients with SCD with relative iron deficiency are more likely to have milder clinical disease.
4 INDUCTION OF IRON RESTRICTION IN PATIENTS WITH SCD
Given the above evidence, strategies that induce a state of relative iron deficiency in patients with SCD may be beneficial. Despite multiple calls for controlled trials to evaluate this hypothesis,23, 53, 56 data are scant and published reports are only observational in nature. To date, the only mechanisms of iron restriction examined in humans have been phlebotomy and apheresis (automated RBC removal without replacement with normal RBCs). These studies are summarized in Table S2.
In 1994, Castro et al. published the effects of inducing iron restriction by periodic RBC removal in two patients with SCD (HbSS).44 One patient underwent RBC removal for approximately 3 years, and he demonstrated a reduction in MCHC and a 23% reduction in HbS polymer fraction (a standardized measure of polymerization occurring at 25% oxygen saturation). Importantly, once started on RBC removal, the patient experienced a marked reduction in the number of days he was hospitalized (approximately 110 inpatient days in the 24 months prior to therapy vs. 16 inpatient days in the 33 months following the initiation of therapy). In contrast, less than 2 months of follow-up data were available for the second patient. He demonstrated a smaller (9%) decrease in HbS polymer fraction and experienced rapid healing of leg ulcers previously resistant to treatment.
There are few additional published data on phlebotomies in patients with HbSS. The studies we now report include patients with HbSC and/or HbSβ-thalassemia, in whom clinical improvement may have been due, at least initially, to a decrease in their hematocrit, rather than to MCHC reduction. In 1995, Rombos et al. described the induction of iron deficiency with weekly phlebotomy in five adults with sickle cell β-thalassemia and a history of painful crises.57 Patients did not exhibit significant changes in mean corpuscular volume (MCV), MCHC, or fetal hemoglobin (HbF) concentration, but it is noteworthy that no patients experienced a vaso-occlusive crisis requiring hospitalization in the year after initiating therapy. This can be contrasted with the average of 3.8 such crises in the year preceding phlebotomy. The use of phlebotomy in a cohort that also included patients with HbSC was described by Bouchaïr et al.46 One patient (with SCA) was an adult, while the six others were pediatric patients (four with HbSC and two with SCA). All experienced frequent vaso-occlusive pain crises, including a total of 144 hospitalized days in the year prior to phlebotomy. In contrast, during the first year of therapy, only 20 hospitalization days were reported for the cohort. The use of phlebotomy across the spectrum of SCD was further evaluated by Rombos et al.58 They performed weekly phlebotomy sessions (removing 100 mL of blood) for 13 patients until ferritin levels reached 10 ng/mL. Although the overall cohort did not exhibit significant reductions in hemoglobin, MCV, or MCHC, a significant reduction in vaso-occlusive crises was observed (Figure 1). Additional support for the use of phlebotomy in HbSC came from a case report by Markham et al.4 The patient, having experienced multiple pain crises attributed to an increased hematocrit following a splenectomy 1 year prior, was started on regular (every 6–8 weeks) phlebotomy to target a hematocrit of 35%. Over a 2-year follow-up period, the patient did not require hospitalization for pain, no longer required opioid pain medication, and exhibited functional improvements.
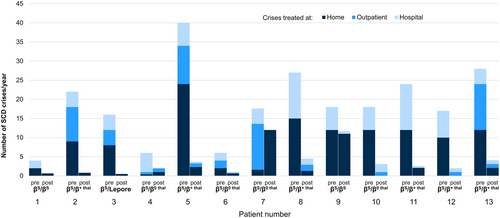
To date, the largest cohort of patients with SCD receiving phlebotomy described in the literature was published by Lionnet et al.59 At a single center treating 179 patients with HbSC, 64 patients qualified for therapeutic phlebotomy because they had hemoglobin values >10.5 g/dL and experienced at least one prespecified complication (i.e., at least one vaso-occlusive crisis requiring hospitalization, at least three vaso-occlusive crises treated in the outpatient setting, priapism, otologic complications, spleen infarct, or arterial thrombosis). Phlebotomy targeted maintenance hemoglobin levels between 9.5 and 10.5 g/dL and was associated with “a good clinical response” (i.e., the absence of pain requiring acute care, a 50% decrease in ambulatory acute vaso-occlusive or priapism episodes, the absence of vestibular syndrome recurrence and/or no aggravation of hypoacousia, and the absence of spleen infarct or recurrence of arterial thrombosis) in 71% of cases.
The most recent published data regarding the use of phlebotomy in patients with SCD come from a cohort of 27 patients (3 with SCA and 24 with HbSC) from Togo.60 Patients had a mean hemoglobin level of 14.3 g/dL and experienced an average of 5.3 vaso-occlusive crises per year prior to initiation of phlebotomy. Treatment was associated with a nearly 2 g/dL drop in hemoglobin levels and an 83% mean reduction in the annual rate of vaso-occlusive crises. No (direct or indirect) measures of iron status were included in the study findings.
The available observational data, although not comprehensive, suggest a beneficial effect of reducing iron via phlebotomy across a spectrum of patients with SCD. Despite inconsistent changes in hematologic parameters, including MCHC, patients treated with phlebotomy consistently demonstrated reduced vaso-occlusive crises. In all reports, phlebotomy was discontinued or held when ferritin or hemoglobin was too low (per treating physicians) to ensure patients remained “safe.” Because a vast majority of patients with HbSC have hemoglobin levels above 10 g/dL,61 phlebotomy may be viewed as more advantageous for this form of SCD. In fact, a proposed treatment algorithm for HbSC recommends that phlebotomy target hemoglobin levels of 9.5–11.0 g/dL and/or a hematocrit of 35% for adults with chronic complications of disease and/or severe acute complications.62 Although randomized controlled data are lacking, in the presence of frequent vaso-occlusive crisis, the judicious use of phlebotomy appears to be a viable treatment option for patients who are only moderately anemic at baseline. Phlebotomy-induced iron restriction can result in both decreased hematocrit and decreased MCHC. For this reason, and because clinical severity in HbSC is partially related to abnormal blood rheology from the higher hematocrit,63 it is likely that phlebotomy-related clinical improvement in this patient group could be due to lower hematocrit, lower MCHC, or both. Clinical outcomes of iron depletion in HbSC will need to be analyzed separately from those in patients with HbSS. In HbSC, clinical improvement could develop as the hematocrit decreases, before the effect of low MCHC is achieved.
The above data should be viewed in the context of multiple limitations. Observational data can only describe association, not causation. In some cases, the studies included historical, rather than prospectively collected, control data. Importantly, in many series, changes in MCHC and ferritin were not reported, making it impossible to assess whether the observed clinical improvement was associated with reduced hematocrit, iron deficiency, or both. A high risk of publication bias is inherent in these results. There is a need for prospectively designed randomized controlled trials with predefined endpoints to fully characterize the therapeutic potential of phlebotomy-induced iron restriction before this strategy is widely employed in clinical practice. In addition to a weak level of evidence supporting its effectiveness, phlebotomy has other limitations. It requires multiple in-person treatment sessions, potentially taxing clinical staff resources and interfering with patient quality of life. Obtaining venous access—particularly in patients with severe disease, many of whom also have poor superficial veins from multiple past venipunctures—can be challenging and might impact patient acceptance of the treatment. Lastly, patients with more severe anemia, including those with chronic kidney disease, would likely be ineligible for phlebotomy. Thus, the use of phlebotomy may not be the optimal method of achieving iron reduction in SCD.
5 ANIMAL MODELS FOR TREATMENT OF SCD WITH IRON RESTRICTION
In a humanized mouse model of SCD, researchers effectively decreased intestinal absorption of iron by disrupting hypoxia-inducible factor (HIF)-2α.47 The disruption of HIF-2α impairs transcription of the gene encoding the intestinal iron transporter divalent metal transporter 1 (DMT1).64 The experimental sickle mice exhibited increased hemoglobin levels, lower serum erythropoietin, and reductions in hemolysis, as assessed by lower bilirubin levels and reticulocyte counts.47
In the last 5 years, several groups have examined the impact of restricting dietary intake of iron in mouse models of SCD. Almashjary et al. initiated Townes SCD (HbSS) mice on either an iron-restricted diet (3 ppm iron) or an iron-normal diet (48 ppm iron) at the time of weaning.65, 66 After 6 months, animals receiving the iron-restricted diet exhibited significantly lower hematocrit levels as well as reductions in hemoglobin and MCV.65, 66 The iron-deficient mice had fewer areas of hepatic necrosis than their iron-replete counterparts, a benefit that could be attributable to improved blood flow.67
In 2021, Parrow et al. examined the effects of modest iron restriction (20 ppm) in sickle trait (HbAS) or SCA (HbSS) mouse models.48 Serum iron concentrations and MCHC were significantly lower in HbSS animals receiving an iron-restricted diet, whereas hematocrit and the number of circulating RBCs were significantly higher (relative to HbSS mice fed standard diets). Notably, HbSS mice on iron-restricted diets exhibited significantly reduced hypoxia-induced sickling using an oxygen gradient ektacytometry assay.
Most recently, the effects of an iron-deficient diet (3 ppm) were compared with those of a diet containing 185 ppm of iron in a murine model of SCD.49, 68 Among animals receiving the iron-restricted diet, reductions in MCHC, markers of hemolysis, vaso-occlusive severity, hepatic necrosis, and liver and spleen weights were all observed (relative to animals receiving a higher dietary intake of iron). Iron restriction was also associated with reductions in intestinal inflammation and gut permeability, which researchers attributed to changes in microbiota induced by the iron-restricted diet. Reduced entry of microbial antigens, including ligands from gram-positive bacteria (toll-like receptor 2 [TLR2] agonists), are hypothesized to contribute to a lower risk of SCD complications.
The studies described above (Table 1) further support iron restriction as a viable therapeutic target for managing SCD. However, decreasing dietary iron is not a feasible/acceptable method for patients with SCD to achieve iron-restricted erythropoiesis.
| Reference | Iron restriction method | MCHC | Hemoglobin level | Hemolysis markers | Improved tissue O2 delivery |
|---|---|---|---|---|---|
| Das et al.47 (2015) | Decreased absorption | Not reported | ↑ | ↓ | Not reported |
| Almashjary et al.65-67 (2019–2020) | Dietary | Unchanged | ↓ | ↓ | Less hepatic necrosis |
| Parrow et al.48 (2021) | Dietary | ↓ | Unchanged | ↓ | Decreased kidney EPO |
| Nyffenegger et al.50 (2022) | Vamifeport (hepcidin mimetic) | ↓ | Unchanged | ↓ | Improved blood flow |
| Li et al.49 (2023) | Dietary | ↓ | Unchanged | ↓ | Improved blood flow, less hepatic necrosis |
- Abbreviations: EPO, erythropoietin; MCHC, mean corpuscular hemoglobin concentration.
Within the last several years, pharmacologic avenues for impacting iron balance, and potentially mimicking the effects of dietary iron restriction and/or phlebotomy, have become available.69 Agents that increase or mimic hepcidin activity may offer a means of restricting iron to the bone marrow, thereby decreasing RBC iron while avoiding the limitations of phlebotomy or dietary restriction. Numerous such agents have been developed and evaluated in vivo (Table S3).70-84 To date, these agents have generally been considered for diseases of iron overload, including β-thalassemia and hereditary hemochromatosis, but they are also undergoing human trials for the treatment of polycythemia vera.
Of the agents that increase hepcidin activity, only vamifeport has been examined in the setting of SCD. Vamifeport is an orally administered small molecule that binds to ferroportin and directly blocks iron export from cells, thus inhibiting dietary iron absorption and iron release from liver and spleen macrophages.83 These actions are expected to induce iron-restricted erythropoiesis. The resultant hypochromic microcytic erythrocytes should have reduced HbS concentrations (MCHC) and be less prone to sickling (Figure 2).50, 83
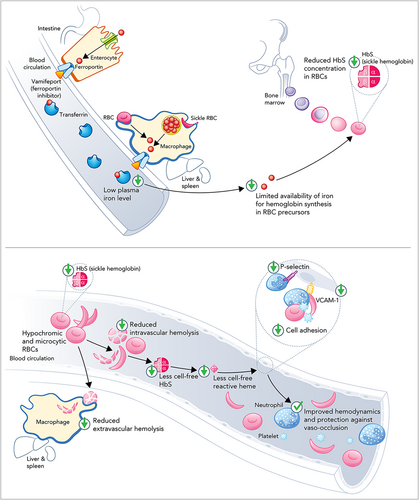
Animal studies appear to support the therapeutic potential of vamifeport in SCD. In the study by Nyffenegger et al., 6- to 7-week-old humanized SCD mice were treated with either vehicle or vamifeport 60 mg/kg (5 mL/kg) twice daily for 6 weeks.50 Vamifeport induced iron-restricted erythropoiesis in the HbSS mice, as evidenced by significantly decreased RBC HbS concentrations (MCHC), MCH, and hematocrit, relative to HbSS animals treated with vehicle. Treatment was not associated with significant changes in RBC counts or the total blood hemoglobin concentration. Significantly lower levels of total heme, LDH, and indirect bilirubin suggest that vamifeport-treated animals experienced less hemolysis than controls. Researchers proposed that improved RBC membrane composition (i.e., reduced phosphatidylserine externalization), increased mitochondria clearance (i.e., 31% reduction in the proportion of mature RBCs containing mitochondria, which may be associated with reduced oxidative stress), and reduced markers of inflammation (e.g., plasma soluble vascular cell adhesion molecule 1 [VCAM-1] and soluble platelet selectin) contributed to the observed decreases in hemolysis.
Given these results, not all the observed decrease in hemolysis may be due to lower MCHC in this model. Some of the favorable effects of iron restriction might be mediated through the reduction of hemolysis and its downstream effects on inflammation and nitric oxide bioavailability. More broadly, iron deficiency is known to activate iron regulatory proteins 1 and 2 (IRP1 and IRP2). Activation of these pathways is known to repress heme synthesis and HIF-2α, resulting in decreased erythropoietin, endothelin-1, and other changes.85 The extent to which IRP1 and IRP2 are activated with iron restriction has not been fully determined.86 An additional potential effect of iron restriction in mice and humans with SCD could be reduced RBC membrane damage from avoidance of iron-related oxidative stress. The absence of published cases of SCD with coinherited variants of iron trafficking genes makes further elucidation of potential mechanisms difficult in the absence of experimental data.
In the animal study by Nyffenegger et al., vamifeport treatment also reduced iron loading in the spleen and liver while improving hemodynamics.50 Notably, the effects of vamifeport were significantly greater than those effects observed in a subgroup of HbSS mice fed a low-iron diet. The data from this study demonstrate that a pharmacologic agent can lead to iron-restricted erythropoiesis and can reduce hemolysis, improve hemodynamics, and decrease inflammation in a mouse model of SCD.50
The results of the above animal studies can be considered “proof of principle” for the benefit of iron restriction in SCA. Nevertheless, only clinical trials will show whether these favorable results translate to net patient improvement. For example, data from β-thalassemia mice showed that inhibition of hemichrome formation improved their hemoglobin level, an effect that has not been replicated in humans.87 Also, and possibly relevant to the translation of mouse data to patients with SCD, murine RBCs are much smaller (40–60 fL) than human RBCs.
6 FURTHER ADVANCING LINCOLN'S THEORY
It has taken 50 years, but we are approaching the point in time when the hypothesis put forth by Lincoln et al. can be rigorously evaluated in patients with SCD. Such studies should evaluate the optimal amount of iron restriction as well as its safety and efficacy, using hematologic, biochemical, and clinical endpoints. The first human study of vamifeport will include 24 patients with βS/β0 or βS/βS genotype.88 This 8-week study will collect safety data and examine the effects of therapy on levels of bilirubin, LDH, potassium, hemoglobin, and free haptoglobin. The results of this randomized, double-blind study will help inform both the future development of the ferroportin inhibitor and validate the clinical utility of iron restriction to improve clinical outcomes in SCD. Initial studies will focus on laboratory markers and surrogates for disease severity, but the effects of iron restriction on clinical events will need to be examined before such therapeutic approaches are embraced in clinical practice. Potential future study endpoints of interest include pain events, tricuspid regurgitation velocity, retinopathy, spleen function, measures of stroke risk (e.g., transcranial Doppler measurements), and lower extremity ulcer progression/improvement.
Currently, little is known about the long-term safety of pharmacologically restricting iron in this population. It is important to balance the benefits of reducing vaso-occlusive events and the risk of iron deficiency anemia. Although it appears that mild iron deficiency anemia is generally well tolerated in patients with SCD, it is not clear what level of hemoglobin will be “too low” to outweigh any benefits conferred by reduced sickling. In animal studies conducted by Parrow et al., iron restriction was associated with a relative decrease in kidney erythropoietin levels, suggesting improved tissue oxygenation despite the lack of improvement in blood oxygen-carrying capacity (hemoglobin level).48 However, induction of iron deficiency in patients with SCD also raises concerns about an increased risk of thrombosis.89-92 Until the effects of this experimental treatment on thrombosis risk are better characterized, it seems prudent to avoid enrolling patients with known thrombophilia or increased baseline risk of thrombosis.
It is not clear from the observational data which individuals with SCD will benefit most from iron restriction. While people with SCA frequently have more severe disease than patients with HbSC, they may not tolerate additional therapy-induced anemia. The absence of HbSC animal models makes it challenging to assess pharmacotherapies in preclinical studies. Thus, small, controlled human studies are needed to examine the safety and efficacy of iron-restriction strategies in this population. It will be important to compare the MCHC reductions achievable by pharmacologic agents with those typically seen in patients with SCD with spontaneous or phlebotomy-induced iron restriction. It will also be necessary to rigorously evaluate the effects of iron restriction across age groups. Because iron is critical for neurodevelopment, and younger patients with SCD are at increased risk for ischemic strokes, caution is warranted when considering an iron-restriction approach in young children. Thus, authors recommend examining the effects of iron restriction on oxygen delivery in adults prior to the initiation of pediatric trials. Because iron is required for fetal development, pregnant women with SCD should not participate in iron deficiency studies. If a woman with SCD becomes pregnant while also iron deficient, iron supplementation should be started, and an intervention such as hydroxyurea should be added. Finally, there are virtually no data examining how pharmacologic iron restriction might interact with other SCD therapies. Future studies should be conducted in populations receiving standard of care therapies.
7 CONCLUSIONS
The available evidence suggests that the induction of iron-restricted erythropoiesis is a promising therapeutic target in the management of SCD. By reducing the HbS concentration within RBCs, this intervention has the potential to reduce sickling and hemolysis, ultimately resulting in fewer vaso-occlusive events. Increased understanding of the hepcidin–ferroportin axis has allowed for pharmacologic induction of iron restriction that moves beyond bloodletting. Although many questions remain, and rigorously designed clinical trials are still needed to further characterize the clinical profile of iron restriction, we are cautiously optimistic that the questions raised by Dr. Lincoln et al. 5 decades ago will be answered affirmatively in the near future.
ACKNOWLEDGMENTS
Editorial and writing assistance provided by Adam Perahia, MD, of NorthStar Strategic Consulting, LLC, and funded by CSL Vifor.
FUNDING INFORMATION
This article is based on discussions at an international advisory board meeting sponsored by CSL Vifor, which is developing therapies for sickle cell disease. CSL Vifor provided funding for writing and editing services to assist with the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
Oswaldo L. Castro: Consultancy: CSL Vifor, Disc Medicine, Inc. Lucia De Franceschi: Consultancy: CSL Vifor, Roche, Novo Nordisk; Research Funding: Agios, Bristol, Afimmune. Tomas Ganz: Scientific Cofounder and Adviser: Intrinsic LifeSciences, Silarus Therapeutics; Consultancy: Akebia, Avidity Bio, Pharmacosmos, Fibrogen, AstraZeneca, Ionis, Gossamer Bio, Global Blood Therapeutics, American Regent, Sierra Oncology, Disc Medicine, RallyBio, Rockwell Scientific, Vifor. Julie Kanter: Honoraria: Medscape, Guidepoint Global, GLG, Cowen, ECOR-1; Consultancy: bluebird bio, Novartis, Fulcrum, Vertex; Scientific Advisory Boards: bluebird bio, Vifor, Novartis, Beam. Gregory J. Kato: Employee of CSL Behring. Sant-Rayn Pasricha: Advisory Board: Vifor Pharma; Consultancy: ITL Biomedical; Research: National Health and Medical Research Council, Australia, Bill and Melinda Gates Foundation, World Health Organization. Stefano Rivella: Scientific Advisory Boards: Ionis Pharmaceuticals, Meira GTx, Incyte, Vifor, Disc Medicine; Stock Options: Disc Medicine; Consultancy: Cambridge Healthcare Research, Celgene Corporation, Catenion, First Manhattan Co., FORMA Therapeutics, Ghost Tree Capital, Keros Therapeutics, Noble insight, Protagonist Therapeutics, Sanofi Aventis U.S., Slingshot Insight, Techspert.io, BVF Partners L.P., Rallybio, LLC, venBio Select LLC. John C. Wood: Research Funding: National Heart, Lung, and Blood Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health; Research Support-in-Kind: Philips Healthcare; Consultancy: Celgene, WorldCare Clinical, Agios, Pharmacosmos, Hillhurst; Advisory Boards: Vifor, Regeneron; Educational Lectures: Chiesi, bluebird bio.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable-no new data generated.




