Carbon monoxide as a clinical marker of hemolysis
Abstract
Carbon monoxide (CO)-based tests have precisely measured hemolysis for over 40 years. End-tidal CO was the primary marker in clinical hematology research, followed by carboxyhemoglobin. Quantification of CO reflects heme oxygenases degrading heme in a 1:1 stoichiometric ratio, making CO a direct marker of hemolysis. CO in alveolar air can be quantified using gas chromatography, whose high resolution allows detecting mild and moderate levels of hemolysis. CO can be elevated in active bleeding, resorbing hematoma, and smoking. Clinical acumen and other markers remain necessary to diagnose the cause of hemolysis. CO-based tests constitute an opportunity for bench-to-bedside technology transfer.
1 WHAT ARE CARBON MONOXIDE-BASED MEASURES?
The clearance of senescent red blood cells (RBCs) is a normal process occurring at the end of a ~120-day lifespan.1, 2 Hemolysis is described as increased and/or early RBC destruction.3 Many diseases and medical conditions can be manifested by increased in vivo hemolysis, such as sickle cell anemia,4 thalassemia,5 hemolytic disease of the newborn (HDN),6 and the use of endoprosthetic devices.7
Several clinical markers help diagnose hemolytic conditions and monitor response to treatment. Each hemolysis marker has characteristics limiting its resolution, such as the specificity of the test, influence of other metabolic pathways, or co-existing medical conditions.8 Current clinical markers detect high levels of hemolysis in vivo but lack in precisely quantifying low-grade hemolysis. This lends the question: what are the most precise markers to identify hemolysis, especially at low levels, and monitor response to treatment?
Lysed RBCs release hemoglobin (Hb) further broken down into heme and globin groups. Heme oxygenases catabolize heme rings to produce biliverdin, ferrous iron, and carbon monoxide (CO).9 Biliverdin is converted to bilirubin in the liver by biliverdin reductase, and correlation between hemolysis and hyperbilirubinemia has been consistently reported.10 Serum iron is a direct marker of heme degradation, while iron carrier proteins ferritin and transferrin are indirect.9, 11 CO produced by heme degradation binds to hemoglobin to form carboxyhemoglobin (COHb).12 As an inert gas, CO is not metabolized, has only one path of elimination through gradient diffusion in the lungs, and is unaffected by other organ dysfunction or disease processes. Hemoglobin heme degradation accounts for approximately 85% of endogenously-produced CO in humans.13 The remaining 15% originates from heme degradation contained in myoglobin, cytochromes, and catalases. These attributes make CO elimination a direct marker of hemolysis.13-15 Several techniques can measure CO, including gas chromatography with reduction gas detection (GC-RGD), gas chromatography with mass spectroscopic detection (GC-MSD), infrared spectroscopy, electrochemical (EC) sensors, spectrophotometric measurement of COHb, [2-14C] glycine to form 14CO, and laser absorption spectroscopy.13, 16 Each technique varies in methods and resolution; for example, GC-RGD uses a heated mercuric oxide bed and high-resolution spectrophotometric sensor to detect small changes in CO (ppb) compared to EC sensors (ppm).13, 14, 16 Besides the resolution, the main differentiating feature between methods is whether CO is being measured from a sample of exhaled air (end-tidal carbon monoxide; ETCO) or blood (COHb). Quantifying endogenously-produced CO requires knowledge of environmental CO levels which will be subtracted from CO measures: CO production = alveolar CO − environmental CO. In this article, we will discuss ETCO using gas chromatography as this technique has the sensitivity and resolution to detect CO changes within the physiological range in humans.
2 IS THERE EVIDENCE TO SUPPORT ITS USE IN CLINICAL HEMATOLOGY?
2.1 A scoping review of the literature
- Which tests are most often used to measure hemolysis in human research?
- Are similar markers used in children as in adults?
- What clinical populations are tested for hemolysis?
We carried out a scoping review that followed the methodological framework developed by Arksey and O'Malley,17 and recommendations from the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis18 and Peer Review of Electronic Search Strategies (PRESS).19 Text words (hemolysis, haemolysis, hemolytic, haemolytic, and measur*) in the title and abstract of relevant citations, as well as index terms, were applied to MEDLINE and EMBASE searches from their inception to June 2021 (see Appendix 1 for search strategy). ClinicalTrials.gov databases were also searched using free text words (hemolysis, assay, sensitiv*, diagnostic accuracy, diagnostic, accuracy). Distiller SR (Evidence Partners Incorporated, Ottawa, Canada) was used to collect citations, remove duplicates, select titles and abstracts, and for full-text revision.20 Two independent reviewers screened citations' titles and abstracts, and extracted the data in a standardized form (Appendix 2).
We identified for each study the primary hemolysis marker and secondary hemolysis markers used as comparators. We presented the results using a summary table of each measurement method and the number of studies that reported them. We also summarized a comparison of hemolysis measurement methods used in children and adults.
2.2 CO-based measures are the primary markers of hemolysis
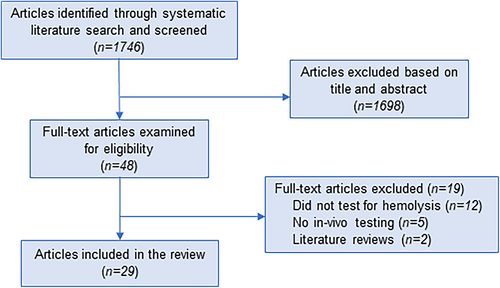
The search strategy identified 1746 articles. These were screened and 48 full-text articles were examined. Animal and in vitro studies were excluded, as were non-English publications and ongoing studies. Twenty-nine articles were included in the review (Figure 1). Articles were published between 1979 and 2021 and included 23 prospective cohort studies, 4 experimental studies of new tests, 1 retrospective chart review, and 1 case–control study. Populations included healthy neonates, children, and adults, as well as individuals with various diseases. Table 1 provides a summary of individual articles.
| Article number | Study | Type of study | Hemolysis marker and measurement method | Comparator | Sample size | Population |
|---|---|---|---|---|---|---|
| 1 | Lozar-Krivec et al. (2021) | Retrospective chart review | COHb via CO-oximeter (Roche-cobas b 221) | Hb, bilirubin; compared populations | 137 | Neonates; healthy, sepsis, HDN, or respiratory distress |
| 2 | Tan et al. (2020) | Experimental—new test | Bilirubin via taper-paper-based sensing device | Bilirubin, Hb | – | Neonates with jaundice; anonymous blood samples |
| 3 | Shahin et al. (2020) | Experimental—new test | ETCO via gas chromatography with reduction gas detection | Numerous previous methods for measuring CO concentrations | 2 | Free from past or current hematological illnesses |
| 4 | Bhatia et al. (2020) | Prospective cohort | ETCO via CoSense device | ARC, bilirubin, Hb | 50 | Newborn infants <35 weeks at risk for hyperbilirubinemia |
| 5 | Zhang et al. (2018) | Case–control | ETCO via ELS TESTER instrument | Alveolar CO | 195 | Children and adults; healthy or chronic hemolytic anemia |
| 6 | Archibong et al. (2016) | Experimental—new test | Free hemoglobin concentration via colorimetric analysis | Healthy ranges of free plasma Hb; compared to other devices | – | Commercially purchased human and bovine plasma and hemoglobin |
| 7 | Sang et al. (2016) | Experimental—new test | Dead RBCs via dielectrophoresis, surface stress biosensor | – | 6 | O+ blood type; healthy or hemolytic anemia |
| 8 | Quinn et al. (2016) | Prospective cohort | ARC and HbF via 15 N-glycine label | AST, LDH, bilirubin, Hb | 11 | Age > 11 years; homozygous sickle cell anemia (HbSS) |
| 9 | Lozar-Krivec et al. (2015) | Prospective cohort | COHb via CO-oximeter (Roche-cobas b 221) | Numerous COHb measurement methods | 86 | Term newborn infants; healthy, ABO HDN, or hyperbilirubinemia without hemolytic disease |
| 10 | Ninomiya et al. (2006) | Prospective cohort | MLS of RBCs via flow cytometry | RBC, Hb, Ht, MCV, reticulocytes, LDH | 6 | PNH |
| 11 | Sylvester et al. (2006) | Prospective cohort | ETCO via CO-Stat End Tidal Breath Analyzer | Bilirubin, ARC, Hb | 33 | Sickle cell disease |
| 12 | Sylvester et al. (2004) | Prospective cohort | ETCO via CO-Stat End Tidal Breath Analyzer | Reticulocytes, COHb, bilirubin, Hb, respiratory rate | 98 | Children; healthy or sickle cell disease |
| 13 | Okumiya et al. (2004) | Prospective cohort | Erythrocyte creatine via enzymatic assay | LDH, ARC, haptoglobin, some cardiac blood flow and cardiac muscle markers | 66 | Healthy or prosthetic cardiac valve(s) |
| 14 | Ho et al. (2003) | Prospective cohort | sTfR and GHb via ELISA technique | Ferritin, Hb, reticulocytes, haptoglobin, LDH, Hb A1c, bilirubin, and other blood markers | 41 | Healthy, hemolytic anemia with effective or ineffective erythropoiesis |
| 15 | Stevenson et al. (2001) | Prospective cohort | ETCO via CO-Stat End Tidal Breath Analyzer, and bilirubin | Coombs' test | 1370 | Healthy neonates |
| 16 | Okuyama et al. (2001) | Prospective cohort | ETCO via Baby's Breath Carbon Monoxide Analyzer | Bilirubin | 51 | Healthy newborn infants |
| 17 | Jiao et al. (2001) | Prospective cohort | Erythrocyte creatine via enzymatic assay | Spleen size, ARC | 100 | Healthy or post-necrotic liver cirrhosis |
| 18 | Burns et al. (2000) | Prospective cohort | EAK via multiple methods | Bilirubin, LDH, ARC, Hb | 99 | Healthy, hemolysis, acute myocardial infarction, or hepatic dysfunction |
| 19 | Chang et al. (1998) | Prospective cohort | ETCO via gas chromatography (GC-8APF machine) | COHb, bilirubin, Hb, ARC | 69 | Age 3–15 years; healthy, B-thalassemia major, or other hemolytic disease |
| 20 | Vreman et al. (1996) | Prospective cohort | ETCO via Baby's Breath Carbon Monoxide Analyzer | Gas chromatography, COHb | 34 | Neonatal ICU patients, healthy smoking adults, or healthy non-smoking adults |
| 21 | Hammerman et al. (1996) | Prospective cohort | COHb via gas chromatography and CO analyzer | Bilirubin, Hb | 26 | Infants; hyperbilirubinemic and Coombs' positive |
| 22 | Vreman et al. (1994) | Prospective cohort | ETCO via EC-CO instrument | Gas chromatography, bilirubin | 108 | 1-day old infants; healthy and non-smoking mothers |
| 23 | Wilke et al. (1992) | Prospective cohort | Haptoglobin via rate nephelometry | Bilirubin, LDH, Hb, peripheral blood smear, numerous other blood markers | 25 | HELLP-syndrome |
| 24 | Ho et al. (1990) | Prospective cohort | GHb via glyco-hemoglobin kit | Hb, Ht, LDH, bilirubin, haptoglobin, ARC | 42 | Cardiovascular or valve diseases undergoing cardiac surgery |
| 25 | Nicholson et al. (1988) | Prospective cohort | COHb via CO-oximeter (IL 282) | Numerous CO measurement methods | 93 | Babies of non-smoking mothers, healthy neonates, or healthy-non-smoking adults |
| 26 | Brouwers et al. (1988) | Prospective cohort | Bilirubin, reticulocytes, other blood markers | ADCC, indirect and direct antiglobulin test, ELISA antigen density | 200 | Newborn infants; ABO-compatible or ABO-incompatible |
| 27 | Smith et al. (1984) | Prospective cohort | ETCO via gas chromatography | COHb, Coombs' test, reticulocytosis, VECO, bilirubin | 23 | Infants; term or pre-term |
| 28 | Napier et al. (1979) | Prospective cohort | 59-Fe via Cavill ferrokinetic technique, and 51-Cr labeling | Hydroxybutyrate, ARC, haptoglobin, urinary hemosiderin, RBC fragmentation, LDH | 10 | Aortic prosthetic heart valve |
| 29 | Fehr and Knob (1979) | Prospective cohort | Erythrocyte creatine via 51-Cr labeling | ARC, 2,3-DPG | 61 | Healthy or steady-state hemolysis/overt hemolytic disease |
- Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity assay; ARC, absolute reticulocyte count; CBC, complete blood count; CO, carbon monoxide; COHb, carboxyhemoglobin; EAK, erythrocyte adenylate kinase; ELISA, enzyme-linked immunosorbent assay; ETCO, end-tidal carbon monoxide; GHb, glycosylated hemoglobin; Hb, hemoglobin; HbF, fetal hemoglobin; HDN, hemolytic disease of the newborn; HELLP, hemolysis, elevated liver enzymes, and low platelets syndrome; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; MLS, mean life span; PNH, paroxysmal nocturnal hemoglobinuria; RR, respiratory rate; sTfR, soluble transferrin receptor; 2,3-DPG, 2,3-diphosphoglyceric acid; 59-Fe, iron-59.
The primary marker of hemolysis was end-tidal carbon monoxide (ETCO) in 11 articles, COHb in 4, erythrocyte creatine in 3, and bilirubin in 2. The remaining nine articles studied a variety of experimental hemolysis markers (Table 2). Primary markers of hemolysis were measured against comparator markers of which bilirubin appeared in 16 articles, hemoglobin in 15, reticulocytes/ARC in 14, LDH in 9, COHb in 6, CO elimination in 4, haptoglobin in 4, and hematocrit in 3. Multiple other comparator markers appeared in one or two articles (Table 2).
| Hemolysis marker | Number of articles appeared as primary hemolysis marker | Number of articles appeared as a comparator hemolysis marker |
|---|---|---|
| ETCO/CO | 11 | 4 |
| COHb | 4 | 6 |
| Erythrocyte creatine | 3 | 0 |
| Bilirubin | 2 | 16 |
| Haptoglobin | 1 | 4 |
| Hemoglobin | 1 | 15 |
| Reticulocytes/ARC | 1 | 14 |
| LDH | 0 | 9 |
| Hematocrit | 0 | 3 |
| Othera | ≤1 | ≤2 |
- Note: Abbreviations are explained in Table 1.
- a Includes: dead RBCs, fetal hemoglobin (HbF), mean life span of RBCs, soluble transferrin receptor (sTfR), glycosylated hemoglobin (GHb), erythrocyte adenylate kinase (EAK), radioactive iron (59-Fe) labeling, Coombs' test, hemosiderinuria, direct antiglobulin test (DAT), ferritin, mean corpuscular volume (MCV), respiratory rate (RR), blood flow velocity, valvular regurgitation, complete blood count (CBC), spleen size, peripheral blood smear, platelets, RBC survival, antibody-dependent cell-mediated cytotoxicity assay (ADCC), indirect antiglobulin test, enzyme-linked immunosorbent assay (ELISA) antigen density, hydroxybutyrate, RBC fragmentation, 24-h urine loss, 2,3-diphosphoglyceric acid (2,3-DPG), D-dimer, schistocyte, duration of phototherapy, RBCs, and general lab markers other than haptoglobin.
There were no significant trends or shifts in hemolysis markers over time. ETCO and COHb represented the primary marker of hemolysis in most articles (n = 15; 52%), consistently, from 1984 to 2021. Electrochemical CO sensors were the primary technology used to measure ETCO, followed by gas chromatography and infrared spectroscopy. CO-oximetry was the primary technology used to measure COHb. CO sensors have appeared consistently in the literature from 1994 to 2020. Gas chromatography appeared in articles from 1984, 1996, 1998, and 2020, infrared technology in 1996, 2001, and 2018, and CO-oximetry in 1988, 2015, and 2021.
2.3 Markers of hemolysis in children and in adults
Of the 11 articles focusing on ETCO as the primary marker of hemolysis, 10 (91%) studied non-adult participants (age < 15). Similarly, all four articles (100%) using COHb as the primary marker of hemolysis were performed on non-adult participants. Of the remaining 14 articles, which focused on a variety of different primary markers of hemolysis, 10 (67%) studied adult participants, 2 (13%) studied neonates, 1 (7%) studied participants >11 years old, and 1 (7%) did not include participants.
2.4 Clinical populations tested for hemolysis
Of the 23 articles studying specific hemolytic conditions, 3 (13%) included participants with sickle cell anemia, 3 (13%) with hemolytic anemia, and 3 (13%) with or at risk for hyperbilirubinemia. Participants with a non-specific hemolytic disease appeared in two (9%) articles, as did participants with HDN, prosthetic cardiac valves, and cirrhosis or hepatic dysfunction. Several other pathological conditions each appeared in one article. Participants admitted to ICU, pre-term neonates, and smoking adults each appeared in one article. Five (22%) articles reported only healthy participants.
3 WHAT ARE ITS ADVANTAGES OVER OTHER HEMOLYSIS MARKERS?
Of hemolysis markers used in clinical hematology research, the measure of CO constituted the most common primary marker of hemolysis across neonate to adult populations, various hemolytic conditions, and in healthy research participants. CO was not the primary marker of hemolysis in articles about cardiac disease, prosthetic cardiac valves, or liver disease.
ETCO and COHb appearing as the primary markers to measure hemolysis in the majority of articles supports the physiology and robustness of CO as a clinical indicator of hemolysis.13-15, 21 Advantages as a marker for hemolysis include that CO is a metabolically inactive molecule and direct stoichiometric marker of heme degradation.12-14 CO has only one path of elimination through gradient diffusion in the lungs and is unaffected by other organ dysfunction or disease processes.12-14 Additionally, ETCO and COHb can be quantitatively measured; ETCO non-invasively through exhaled air, while COHb measurement requires a blood sample. These advantages and evidence from this review qualify CO as a gold standard to precisely measure hemolysis.
Other hemolysis markers do not possess these attributes. Bilirubin is included in the clinical work-up for suspected hemolysis, and as a marker of response to treatment.22 However, its usefulness is reduced because hyperbilirubinemia is associated with several non-hematologic medical conditions (e.g., gallstones, cholangitis, Gilbert's syndrome, and liver cirrhosis).23 Free Hb is a semiquantitative marker of hemolysis because altered levels can be attributed to several different metabolic pathways and diseases (e.g., various types of anemia),22, 24 and it is largely dependent on haptoglobin availability. Free Hb can be elevated in intravascular hemolysis (RBCs destroyed within vessels), differentiating it from extravascular hemolysis (RBCs phagocytosed in the monocyte–macrophage system).25 Haptoglobin itself as a marker of hemolysis has limited usefulness because it is affected by co-existing medical conditions (e.g., malnutrition, liver cirrhosis, hemodilution, hypersplenism, and certain medications).22, 24 Similarly, elevated serum LDH is not specific to a hemolytic process as it can indicate infection, tissue damage or necrosis (e.g., liver, muscle, and bone), and vitamin deficiencies (e.g., vitamin B12 and folate).22, 26 Serum iron is a direct marker of hemoglobin heme degradation, however, can be affected by bleeding, dietary deficiencies, and various anemias that limit its precision as a measurement of hemolysis.9, 27 Iron transporter proteins, transferrin and ferritin, are indirect markers of heme degradation that are also modulated in various conditions, including infection, cancer, autoimmune disorders, and chronic kidney disease.22, 28 Additionally, blood transfusions, common in patients with hemolysis, can increase total body iron.22 Reticulocytes indicate the bone marrow erythropoietic response to hemolysis,22 and like bilirubin and hemoglobin, assess response to therapy. However, various factors influence reticulocyte production including anemia, blood loss, altitude, and environmental oxygen levels.22 Haptoglobin, bilirubin, free Hb, LDH, and reticulocytes are affected by various physiologic pathways and medical conditions, thus decreasing their validity to accurately quantify hemolysis.10, 22, 26, 29, 30 A thorough clinical work-up for hemolysis may include an array of markers, which together may identify increased hemolysis; however, no individual marker appears to be accurate enough to precisely quantify hemolysis, especially at low levels.
4 WHAT ARE ITS LIMITATIONS AND PITFALLS?
Previous authors recommended the clinical implementation of ETCO to quantify hemolysis,13, 21, 31 and these recommendations were followed by many neonatal units.32, 33 Despite being the most studied marker of hemolysis and possessing advantages over other markers, CO elimination testing has not been widely accepted as a standard measurement of hemolysis in most clinical settings and patient demographics. This constitutes a large divide between evidence-based literature and clinical practice. The reasons for the divide, persistent over the past 40 years, are unclear. Current clinically designed CO-measuring devices are non-invasive, fast, and can be performed on patients of all ages and varying disease states. Some commercially available devices lack resolution in the human physiological range to detect mild or moderate hemolysis, thus deterring their use clinically. Research laboratory methods can overcome these limitations, but are rarely used outside of experimental settings.25 The use of CO as a hemolysis marker appears to show wider penetration in pediatric clinical settings compared to adult populations,32-34 which may reflect more active clinical research to test and diagnose genetic hemolytic conditions in neonates and children compared to acquired causes in adult populations.
CO can accurately diagnose and quantify hemolysis but bears limitations for clinical use. CO concentrations can be spuriously increased in cases of active bleeding, resorbing hematoma, and in the presence of exogenous sources of incomplete combustion products such as smoking and air pollution.35, 36 The most obvious limitation of CO, however, is its inability to differentiate various causes of hemolysis. For patients, it is important to distinguish between congenital and acquired hemolysis, acute and chronic, and intravascular and extravascular, and to do so requires a comprehensive evaluation with multiple markers. Even considering the confounders of less specific markers, they are fundamental in working up a clinical diagnosis and implementing targeted treatment. Specifically, ETCO and COHb cannot discriminate between intravascular and extravascular hemolysis, which is significant because the same degree of intravascular and extravascular hemolysis can have different clinical outcomes. Extravascular hemolysis typically occurs at a rate ~10-fold less than intravascular hemolysis.22 Theoretically, CO measures could predict that lower levels of hemolysis likely correlate with extravascular hemolysis; however, hemolytic patients would benefit from complete work-up for diagnostic accuracy. This could include haptoglobin, LDH, and hemosiderin, which accompany intravascular hemolysis; hemoglobin and reticulocytes to assess bone marrow contribution to compensated or uncompensated hemolysis; and morphology analysis via peripheral RBC smear to diagnose RBC membrane defects.22
5 AN OPPORTUNITY FOR TECHNOLOGY TRANSFER?
Current clinical markers can identify hemolysis levels manifold larger than physiological hemolysis; however, mild and moderate hemolysis may remain invisible and missed. Normative hemolysis generates approximately 1500 parts per billion (ppb) of end-tidal CO.14 To detect a 20% increase, a measure should reliably identify a rise of 300 ppb. These are outside the realm of protein-based markers but within the range of CO-based methods. For example, we used precise CO methods and measured, in clinical studies of small sample sizes, hemolysis increased by 23% and 54% in patients confined to bedrest and in microgravity in space, respectively.11, 37 Previous studies using CO identified increases of 25% in patients with rheumatoid arthritis,38 endoprostheses,39 or hepatitis C.40 These levels of increased hemolysis can be missed by other markers. In addition to these conditions, it was suggested that increased hemolysis complicates kidney disease,41 ICU stays,42 and possibly aging, where one-third of all geriatric anemia remains unexplained.43 At the epidemiological level, CO-based methods may identify large populations with unsuspected low-grade hemolysis, potentially improving their hematological outcomes.
The divide in the clinical measure of hemolysis constitutes an opportunity for healthcare technology transfer. Bringing to hospital practice, the most precise measure of hemolysis would bridge this divide and improve the care of patients with low and moderate levels of hemolysis. Standardized CO-based measurement could also involve cost-saving benefits by decreasing the number of imprecise tests performed. These data support implementing cost–benefit analyses for healthcare facilities followed by large-scale clinical studies using CO-based methods for the measure of hemolysis.
New technologies, such as CO-specific biochemical sensors44-47 and fluorescent probes,48-53 have the potential not only to remove CO during intoxication, but also to quantify CO in tissues and track the distribution of CO in vivo. These advancements, while tested primarily on animals, represent exciting progress in developing more precise clinical measurement of CO.
6 CONCLUSIONS
Precisely measuring hemolysis is clinically important for a variety of hematological and chronic diseases. CO-based methods surpass other clinical markers as a direct measure of hemolysis, not influenced by most co-morbidities, not metabolized from production to elimination, and quantitative in the human physiological range. Despite being reliably used to study hemolysis for over 40 years, these advantages have not benefitted clinical practice. This article identified a divide between research on hemolysis and clinical practice, which constitutes an opportunity for bench-to-bedside technology transfer to improve the screening, diagnosis, treatment monitoring, and long-term management of patients of all ages with low levels of hemolysis.
6.1 Ethics statement
The final review was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Review guidelines.54 The results provide an overview of the current measurement methods available for hemolysis. It provides information for key stakeholders: researchers, physicians, governments, and public health agencies describing efficacies and limitations of the studied tests, as well as potential gaps in the literature. The scoping review also helps in the planning of future economic analyses. Research ethics approval is not necessary for this study.
ACKNOWLEDGMENTS
This study was supported in part by an Excelerator grant from the Blueprint Translational Research Group of the University of Ottawa to Guy Trudel. Other collaborators include Zeinab Daham who screened citations' titles and abstracts, and Dean Fergusson who edited the manuscript.
FUNDING INFORMATION
This study was supported in part by an Excelerator grant from the Blueprint Translational Research Group of the University of Ottawa to Guy Trudel. This funding source had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit results.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Appendix 1: COMPLETE SEARCH STRATEGY.
- (hemolysis or haemolysis or hemolytic or haemolytic).mp. and measur*.tw,kf. (25560).
- (sensitiv* or predictive value*).mp. or accurac*.tw. (5045056).
- Reproducibility of Results/ (580627).
- 2 or 3 (5366906).
- 1 and 4 (3645).
- ([hemolysis or haemolysis or hemolytic or haemolytic] and measur*).ti. and (“in data review” or in process or publisher or “pubmed not medline”).st. (13).
- 5 or 6 (3653).
- exp animals/ not humans/ (19277937).
- 7 not 8 (2468).
- 9 use medall (1249).
- *hemolysis assay/ or *hemolysis/ (25479).
- (hemolysis or haemolysis or hemolytic or haemolytic).tw. (198575).
- 11 or 12 (204148).
- measur*.tw. (8512834).
- 13 and 14 (22083).
- sensitiv*.tw. or diagnostic accuracy.sh. or diagnostic.tw. or accuracy.tw. (5602600).
- reproducibility/ (229663).
- 16 or 17 (5761007).
- 15 and 18 (3279).
- exp animals/ not exp humans/ (10391333).
- 19 not 20 (2853).
- conference abstract.pt. (4069719).
- 21 not 22 (2210).
- 23 use emczd (1258).
- 10 or 24 (2507).
- remove duplicates from 25 (1781).
- 26 use medall (1247).
- 26 use emczd (534).
Only English language publications were used. Animal, in vitro, and ongoing studies were excluded.
Appendix 2: DATA EXTRACTION FORM.
| Ref ID | Title | Authors | Published year | Journal | Country of origin | Type of study | Funding | Hemolysis measurement method | Comparator standard | Patients involved (Y/N) | Sample size | Population | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | Clinical factors influencing endogenous carbon monoxide production and carboxyhemoglobin levels in neonates | Jana Lozar Krivec, Katja Lozar Manfreda, Darja Paro-Panjan | 2021 | Journal of Pediatric Hematology/Oncology | Slovenia | Retrospective chart review | – | COHb (carboxyhemoglobin) via point-of-care blood gas analyzer (Cobas b 221 system, Roche Diagnostics GmbH, Germany) | Hb, bilirubin; compared neonates with hemolysis to healthy neonates or neonates with sepsis and respiratory distress within study population | Y | 137 | Neonates; four groups: positive blood cultures or clinical sepsis, respiratory distress of different etiologies, hemolytic disease of the newborn or various etiologies, healthy neonates | The group with hemolysis had the lowest mean age at COHb measurement, the highest median COHb and mean bilirubin, and the lowest mean Hb values; according to the model, having hemolysis had the largest influence on COHb values, followed by postnatal age at time of COHb measurement and Hb value, while sex had no statistically significant effect on the COHb value; ROC curve of sensitivity determined the accuracy and optimal COHb cut-off value for confirming hemolysis (95% confidence interval, optimal COHb cut-off value >1.7%) | COHb can be used to detect hemolysis since COHb levels were significantly higher in neonates with hemolysis; ROC curve confirms high diagnostic accuracy |
| 21 | Three-dimensional microfluidic tape-paper-based sensing device for blood total bilirubin measurement in jaundiced neonates | Weirui Tan, Liyuan Zhang, James C. G. Doery, Wei Shen | 2020 | Lab on a Chip | Australia | Experimental—new test | Monash Institute of Medical Engineering (MIME); MIME-SPARK; Australian Research Council through Research Hub for Energy Efficient Separation Project and Australian Research Council Grant; Department of Industry, Innovation and Science through Innovation Connections Projects | Tape-paper-based sensing device for total bilirubin; caffeine-benzoate paper and serum bilirubin sample | Bilirubin, Hb; Monash Children's Hospital Laboratory method for determining total bilirubin | N | - | - | Caffeine-benzoate is an accelerating reagent for unconjugated bilirubin diazotization; the tape-paper sensor (compared to a paper sensor) efficiently suppresses the lateral chromatographic elution effect, reduces water evaporation (important driving force for creating the coffee ring), and the color development in the detection zone can be scanned while wet which enables a higher color intensity to be recorded; this 3D microfluidic taper-paper-based sensing device realizes both plasma separation and detection of total bilirubin; the measured color intensity of the standard threshold bilirubin concentration using the tape-paper sensor can be employed to compare with that of the real sample, and if the color intensity of a real sample is weaker than that of the threshold color reading, the bilirubin level then can be considered safe and vice versa; the tape-paper sensing approach for quantification of blood total bilirubin has negligible interference from hemoglobin; the tape-paper sensing method is accurate and reliable to quantify the total bilirubin level in jaundiced human blood | The novel tape-paper sensor could generate uniform color distribution on the paper substrate by eliminating the coffee stain effect; the interference study confirms that hemoglobin level < 100 mg/dL has a negligible interference effect on the bilirubin quantification; the accuracy of the tape-paper sensing approach for neonatal total bilirubin measurement was validated by the hospital laboratory method; the novel device can be used to screen the bilirubin level in an infant whether below or above the threshold concentration based on the color intensity comparison; the tape-paper sensor consumes minimal volume of reagents and blood samples; detection is fast (<10 min) and relies on minimal equipment; the device has potential to be commercialized for POC diagnosis of neonatal jaundice |
| 26 | Measuring human hemolysis clinically and in extreme environments using endogenous carbon monoxide elimination | Nibras Shahin, Hakim Louati, Guy Trudel | 2020 | Annals of Biomedical Engineering | Canada | Experimental—new test | Canadian Space Agency Contracts and Grant | Endogenous CO elimination via gas chromatograph with mercuric oxide reduction gas detector | Previous methods for measuring alveolar and ambient CO concentrations, carboxyhemoglobin, end tidal air CO concentration, reticulocyte count, presence of schistocytes, presence of free Hb, haptoglobin level, bilirubin and indirect bilirubin level, ferritin level, LDH level, d-dimers, presence of hemosiderinuria, direct antiglobulin test | Y | 2 | Free from past or current hematological illnesses, on no medication | CO elimination is maximal at noon, lowest at midnight; negligible effect of stored CO up to 11 months; the 2-point calibration and interpolation steps increased accuracy, and the data demonstrated precise and accurate measures of gases of known CO concentration; subtracting ambient CO concentration from alveolar CO concentration improves the validity and accuracy of this CO elimination method to more directly reflect human steady state hemolysis; CO elimination methods are the quantitative gold standard for clinical research on hemolytic diseases | Methods demonstrate accurate measures of the rate of human RBC destruction/hemolysis; methods with increased accuracy for adult clinical use to complement and augment existing methods; portable method for remote or extreme environments; non-invasive yet robust measure of hemolysis rate; CO is the product of a unique degradation pathway, unlike other tests that are direct markers of hemolysis |
| 92 | Noninvasive detection of hemolysis with ETCOc measurement in neonates at risk for significant hyperbilirubinemia | Ashwani Bhatia, Mei Chien Chua, Rowena dela Puerta, Victor Samuel Rajadurai | 2020 | Neonatology | Singapore | Prospective cohort | – | CoSense device; breath ETCOc (end-tidal carbon monoxide corrected to ambient carbon monoxide) | Reticulocyte count, serum bilirubin, duration of phototherapy, mean hemoglobin | Y | 50 | Newborn infants, <35 weeks old, birth weight > 2000 g, with G6PD deficiency, blood group incompatibility, or clinical jaundice needing phototherapy during first 7 days of life; infants at risk for hyperbilirubinemia | Mean ETCOc was 1.61 (±0.56) ppm; linear correlation between increasing ETCOc values and reticulocyte counts; 16 newborns with ABO incompatibility had a significantly higher mean ETCOc of 1.98 ppm (±0.71) as compared to 1.43 (±0.38) ppm in the nonhemolytic hyperbilirubinemia group (n = 25); this was suggestive of hemolysis as shown by the significantly higher reticulocyte count of 6.90% (±3.38) compared to 4.68 (±1.26) in the nonhemolytic hyperbilirubinemia group; neonates with an ETCOc level ≥ 1.8 ppm had a higher reticulocyte count, lower hemoglobin level, higher serum bilirubin levels, rapid rise in serum bilirubin, and needed a longer duration of phototherapy; ETCOc values ≥1.8 ppm were suggestive of hemolysis (reticulocyte count ≥6%), with a sensitivity of 90% and a specificity of 83% | Higher ETCOc values ≥1.8 ppm are suggestive of hemolysis and are associated with significant hyperbilirubinemia; lower ETCOc values <1.8 ppm are suggestive of the absence of active hemolysis and a decreased likelihood of developing significant hyperbilirubinemia; in infants with ABO incompatibility, lower ETCOc values may be reassuring and obviate the need for unnecessary phototherapy by using the normal-risk category instead of the high-risk category; ETCOc is a simple, non-invasive, POC test |
| 159 | Human erythrocyte lifespan measured by Levitt's CO breath test with newly developed automatic instrument | Hou-De Zhang, Yong-Jian Ma, Qi-Fa Liu, Tie-Zhen Ye, Fan-Yi Meng, Yi-Wen Zhou, Guo-Pan Yu, Jian-Ping Yang, Hua Jiang, Quan-Shi Wang, Gui-Ping Li, Yong-Qiang Ji, Guo-Liang Zhu, Li-Tao Du, Kun-Mei Ji | 2018 | Journal of Breath Research | China | Case–control—testing reliability of a test with a new instrument | Innovation Fund for Technology Based Firms, the Ministry of Science and Technology, PR China | ELS TESTER instrument, Levitt's CO breath test, new automatic instrument to detect abnormally short RBC lifespan (ELS TESTER, Seekya Biotec Co. Ltd, Shenzhen, China) | Alveolar sample CO, endogenous alveolar CO | Y | 195 | 104 healthy subjects (46 children, 58 adults), 91 patients with chronic hemolytic anemia | In healthy subjects, the RBC lifespan was 126 ± 26 days, similar to values obtained with classical standard labeling methods; RBC lifespan did not differ significantly between males and females or between juveniles and adults, and did not correlate with age; in subjects with hemolytic anemia, RBC lifespan was 29 ± 14 days, which is significantly shorter than that of the healthy subjects; using 75 days as a cut-off, diagnostic accuracy for hemolytic anemia was 100%; mean alveolar sample CO2 concentration of 104 healthy subjects was 5.8 ± 0.6%, and that of 91patients with hemolytic anemia was 6.1 ± 2.5%; mean endogenous alveolar CO concentration was 1.8 ± 0.5 ppm in healthy subjects and 5.9 ± 3.2 ppm in patients with hemolytic anemia; new ELS TESTER instrument determines alveolar endogenous CO concentration by non-dispersive infrared spectroscopy with paired alveolar and air gas samples, and uses that measurement as a basis for determining RBC lifespan with Levitt's formula | Levitt's CO breath test is an ideal method for human RBC lifespan measurement, and the newly developed automatic instrument is reliable and convenient for clinical practice; normal-range RBC lifespans in healthy subjects and reduced RBC lifespans in patients with hemolytic anemia; simple, rapid, and accurate CO breath test methodology based on Levitt's CO analysis principle can enable RBC measurements to be taken with ease in clinical settings |
| 285 | A mobile phone-based approach to detection of hemolysis | Edikan Archibong, Karthik Raj Konnaiyan, Howard Kaplan, Anna Pyayt | 2016 | Biosensors and Bioelectronics | USA | Experimental—new test | National Science Foundation, I-CORPS Hemolix—Technology for Detection of Pregnancy Complications | Mobile phone-based hemolysis-measurement platform (Hemolix); mobile phone camera performs a colorimetric analysis of free hemoglobin concentrations | Compared to healthy ranges of free plasma hemoglobin; compared to other devices Roche Cobas c501 and Siemens Dimension Vista 1500 | N | - | - | Findings suggests that 5 min settling time is adequate to obtain several millimeters of imaginable plasma; intensity of the red value (a) is directly proportional to hemoglobin concentrations over the range 0–50 mg/dL, but reaches saturation at concentrations >50 mg/dL; for the channel corresponding to the concentration of proteins in the plasma sample (yellow coloration, b values), the intensity is inversely proportional to hemoglobin concentration; the normalized ratio of all samples to the output values of the blank sample (plasma) increases as the hemoglobin concentration increases; intensity of parameter L (lightness) is also generally inversely proportional to the concentration of free hemoglobin; hemoglobin concentrations can be measured with an accuracy of ~1 mg/dL at lower hemoglobin values; this level of accuracy is sufficient to reliably differentiate the qualitative and quantitative concentration categories: <5 mg/dL for non-hemolyzed, 5–30 mg/dL for slightly hemolyzed, 30–60 mg/dL for mildly hemolyzed, 60–300 mg/dL for rankly hemolyzed, and > 300 mg/dL for grossly hemolyzed; examples of accuracies reported for other systems are 13 mg/dL and 10 mg/dL (Roche Cobas c501 and Siemens Dimension Vista 1500, respectively) | Low-cost, point-of-care detection of hemolysis; blood plasma is separated from the whole blood using gravitational sedimentation and thus can be done promptly without centrifugation; Lab colors pace can differentiate varying concentrations of hemoglobin in the blood plasma while also identifying samples with interfering levels of bilirubin; Android application provides ultra-fast digital image processing; LOD of hemoglobin concentration with the mobile device was found to be 1.39 mg/dL, which is within the reference limit of cell-free hemoglobin within plasma; this approach can significantly reduce errors associated with subjective visual methods and can shorten the analysis time relative to that required by standard automated techniques |
| 300 | Portable microsystem integrates multifunctional dielectrophoresis manipulations and a surface stress biosensor to detect red blood cells for hemolytic anemia | Shengbo Sang, Qiliang Feng, Aoqun Jian, Huiming Li, Jianlong Ji, Qianqian Duan, Wendong Zhang, Tao Wang | 2016 | Scientific Reports (Open) | China | Experimental—new test | National Natural Science Foundation of China, Doctoral Fund of MOE of China, 863 project, Shanxi Province Foundation for Youths | Portable multifunctional dielectrophoresis manipulations device and a surface stress biosensor to separate and detect red blood cells; content of dead RBCs, diagnosis of hemolytic anemia by electric signal | - | Y | 6 | 1 healthy, 5 with hemolytic anemia; all O+ type blood | After manipulating and separating the living/dead cells mixture, the Re(KCM) is 0 for living cells and close to 1 for dead cells; the living/dead blood cells can be detected according to their capacitance values; the surface stress induced by the dead RBCs decreases due to the destruction of the molecular structure; the capacitance of dead RBCs reached a steady state with a larger capacitance than the living RBCs; healthy blood cells showed lower final capacitance values (5.90 pF); the capacitance signals were different for the five lines (31.24 pF, 28.69 pF, 23.80 pF, 22.08 pF, and 20.01 pF), which indicate different degrees of hemolytic anemia for the patients | Hemolytic anemia can be diagnosed by monitoring output capacitance; this portable microsystem can be used to diagnose the state of hemolytic anemia by the detection of blood red cells; unique electrode configuration creates a highly non-uniform electric field to significantly improve sensitivity; the integrated surface stress biosensor can quantitatively detect the sorted RBCs by the variation in the capacitance values; the portable microsystem can not only manipulate and separate living/dead RBCs effectively, but also detect living/dead RBCs |
| 305 | Biochemical surrogate markers of hemolysis do not correlate with directly measured erythrocyte survival in sickle cell anemia | Charles T. Quinn, Eric P. Smith, Shahriar Arbabi, Paramjit K. Khera, Christopher J. Lindsell, Omar Niss, Clinton H. Joiner, Robert S. Franco, Robert M. Cohen | 2016 | American Journal of Hematology | USA | Prospective cohort | NIH Clinical and Translational Science Award (CTSA) program, NIH-NHLBI Excellence in Hemoglobinopathy Research Award, VA Merit Award | RBC survival, HbF, absolute reticulocyte count (ARC); oral 15 N-glycine age cohort label to measure RBC survival in individuals with sickle cell anemia (HbSS) | Biochemical surrogate markers of hemolysis, AST, LDH, total bilirubin, indirect bilirubin, and plasma free hemoglobin | Y | 11 | Diagnosed homozygous sickle cell anemia (HbSS), age > 11 years, no medical issues needing acute intervention in the 1 month prior to enrollment | Mean RBC survival was 31.9 days (S.D. 12.0 days) with a range of 14.1–53.6 days; mean RBC survival was shortened in study participants by 50%–85% compared to normal controls; mean RBC survival had a strong inverse correlation with absolute reticulocyte count and percentage of reticulocytes; the coefficients of determination for absolute reticulocyte count (ARC) and percentage of reticulocytes were 0.71 and 0.61 respectively; positive correlation between mean RBC survival and percent HbF; every 1% increase in HbF was associated with a 1.9-day increase in mean RBC survival; no significant correlations were observed between mean RBC survival and any other complete blood count parameter; participants with 2-gene-deletiona-thalassemia had longer mean RBC survival than would be predicted from HbF level alone; commonly used biochemical surrogate markers of hemolysis, AST, LDH, total bilirubin, indirect bilirubin, and plasma free hemoglobin, had no significant correlation with directly measured RBC survival | RBC labeling with orally administered 15 N-glycine is a safe and practicable method to measure RBC survival directly, and thereby quantify total hemolysis; commonly used biochemical surrogate markers of hemolytic rate (LDH, AST, bilirubin, and plasma free hemoglobin) do not correlate with directly measured RBC survival; ARC and HbF level did correlate with directly measured RBC survival; ARC was the best correlate of total hemolysis, but only 70% of the variation in RBC survival was reflected in this marker |
| 312 | The role of carboxyhemoglobin measured with CO-oximetry in the detection of hemolysis in newborns with ABO alloimmunization | Jana Lozar-Krivec, Borut Bratanic, Darja Paro-Panjan | 2015 | Journal of Maternal-Fetal and Neonatal Medicine | Slovenia | Prospective cohort | – | COHb measured with CO-oximeter (Roche-cobas b 221); COHb, bilirubin, Hb | Compared COHb measurement methods (gas chromatography most precise method, but complex and time-consuming); mean COHb value determined with gas chromatography in infants with RhD HDN is reported to be between 2.13 and 2.93%, which is comparable to the median COHb value (2.43%) in Group 1 in our study | Y | 86 | Term newborn infants; 18 with ABO HDN (hemolytic disease of the newborn), 21 with hyperbilirubinemia without hemolytic disease who required phototherapy, 47 healthy controls | The ABO HDN infants had significantly higher COHb values than the healthy controls (median 2.4% vs. 1.3%) and the group with hyperbilirubinemia without hemolytic disease (median 2.4% vs. 1.3%), although the infants with hyperbilirubinemia without hemolytic disease did not have significantly higher COHb values compared with the healthy controls; the cut-off value of 1.7% COHb had 72% sensitivity and 97% specificity for confirming hemolysis in ABO alloimmunization | COHb values determined with CO-oximeters are higher in newborns with hemolysis than in those without hemolysis; COHb measured with CO-oximeters could be used to confirm hemolysis in infants with ABO alloimmunization; CO-oximetry method is not optimal for assessing small differences in COHb, it is sufficiently reliable to confirm hemolysis in ABO alloimmunization |
| 700 | Shortened lifespan of paroxysmal nocturnal haemoglobinuria-affected RBC estimated from differences in ratios of CD59-negative populations between reticulocytes and whole RBC | H. Ninomiya, S. Sato, Y. Hasegawa, T. Nagasawa | 2006 | International Journal of Laboratory Hematology | Japan | Prospective cohort | – | Estimated/calculated via formula mean lifespan (MLS) of PNH-RBC from the differences in ratios of CD59-negative populations between reticulocytes and whole RBCs; used reticulocyte-gated two-color flow cytometry (RBC stained with CD4K530 dye on FACSort) | Other data included RBC counts, Hb, Ht, MCV, reticulocyte %, lactate dehydrogenase | Y | 6 | Paroxysmal nocturnal haemoglobinuria (PNH), diagnosed from positive tests for complement-sensitive RBC and from significant sizes of CD55- and CD59-negative PNH populations in RBCs and leucocytes | MLS values of PNH-RBC estimated from the formula were distributed from 16 to 45 days; there was a weak positive correlation between RBC and reticulocyte numbers; however, in individual PNH patients, the reticulocyte numbers changed without any relation to the degree of anemia, suggesting that the erythropoietic response to chronic anemia does not always result in a recovery from anemia in PNH, probably due to hemolysis; MLS of PNH-RBC showed a weak positive and a weak negative relation with RBC and percentage reticulocytes, respectively; a large degree of PNH-affected erythropoiesis was not always associated with severe anemia | MLS of PNH-RBC estimated by our methods could be a useful parameter for evaluating clinical hemolytic conditions quantitatively; weak positive correlation between MLS and the RBC count, suggesting that a short MLS is one of the factors affecting the degree of anemia in addition to the ratio of CD59-negative erythropoiesis and total erythropoietic activity; degree of anemia is determined by several factors, one of which is an MLS different among patients, determined and altered by peripheral conditions promoting hemolysis; this method enables the detection of a small PNH-affected erythropoiesis and is useful for following up PNH patients; MLS estimated by these methods mainly reflect the hemolytic conditions in vivo before the assay; no correlation of the MLS of PNH-RBC with other hemolytic parameters represented by lactose dehydrogenase, the estimation of MLS using this formula is beneficial for the non-use of radioisotopes and for repeatability during the clinical course of PNH |
| 765 | Computed tomography and pulmonary function abnormalities in sickle cell disease | K.P. Sylvester, S.R. Desai, A.U. Wells, D.M. Hansell, M. Awogbade, S.L. Thein, A. Greenough | 2006 | European Respiratory Journal | UK | Prospective cohort | Amanda Smith Charitable Trust, Development Grant from the Medical Research Council, Pump Priming Grant from the Royal College of Radiologists | End tidal carbon monoxide (ETCO), and its correlation with pulmonary function abnormalities (lung volumes, spirometry, gas transfer, oxygen saturation) and high-resolution computer tomography (HRCT; degree of lobar volume loss, ground-glass opacification, prominence of central vessels) in sickle cell disease (SCD); ETCO levels measured using an ETCO monitor (CO-Stat) | Other data included total bilirubin, absolute reticulocyte, hemoglobin levels | Y | 33 | Sickle cell disease (SCD) | ETCO levels significantly negatively correlated with FEV1, vital capacity measured using a plethysmograph, specific airway conductance and arterial oxygen saturation measured by pulse oximetry; forced expiratory volume in one second (FEV1), forced vital capacity and total lung capacity significantly correlated with HRCT findings, particularly lobar volume loss; ETCO levels correlated positively with bilirubin levels, absolute reticulocyte count, and negatively with hemoglobin; ETCO levels correlated negatively with FEV1, VCpleth, specific airway conductance, and arterial oxygen saturation measured by pulse oximetry | Results suggest that high-resolution computed tomography noninvasive assessment of hemolysis might be useful to identify sickle cell disease patients with respiratory function impairment; ETCO levels reflected the degree of hemolysis in SCD adults, as assessed by the total bilirubin, hemoglobin levels and reticulocyte count; significant relationship between pulmonary function and ETCO levels is not surprising, as a correlation between hemolysis and pulmonary function in SCD would be expected; finding that ETCO levels negatively correlate with lung function results in SCD patients suggest that this noninvasive assessment of hemolysis might be useful to identify SCD patients with lung function abnormalities |
| 807 | A novel device for the non-invasive measurement of free hemoglobin in blood bags | Karl P. Sylvester, Richard A. Patey, Gerrard F. Rafferty, David Rees, Swee Lay Thein | 2004 | European Journal of Pediatrics | UK | Prospective cohort | Community Fund and Well Child Trust Medical Research Fund | ETCOc (corrected), using an electro-chemical sensor (CO-Stat, Natus Medical Inc) | Other data included reticulocyte levels, carboxyhemoglobin, bilirubin, hemoglobin, respiratory rate | Y | 98 | 72 children with SCD, 26 age and ethnic origin matched healthy controls | Positive correlations between the ETCOc and carboxyhemoglobin and bilirubin levels, and a significant negative correlation between the ETCOc and hemoglobin levels; the mean and SD ETCOc levels of the SCD children (4.9 ppm; SD 1.7 ppm) were significantly higher than that of the controls (mean 1.3 ppm; SD 0.4 ppm); | Measurement of end-tidal carbon monoxide levels is a reliable and useful method to monitor hemolysis in children with sickle cell disease; ETCOc levels measured in SCD children reflect hemolysis, as significant positive correlations were demonstrated between ETCOc and both carboxyhemoglobin and bilirubin levels, and a significant negative correlation with hemoglobin level; this technique may be useful to monitor children with SCD at risk of complications due to high levels of hemolysis |
| 858 | Evaluation of intravascular hemolysis with erythrocyte creatine in patients with cardiac valve prostheses | Toshika Okumiya, Mitsuko Ishikawa-Nishi, Tadafumi Doi, Mikio Kamioka, Hiroaki Takeuchi, Yoshinori Doi, Tetsuro Sugiura | 2004 | CHEST Journal | Japan | Prospective cohort | Japan Society for the Promotion of Science | Erythrocyte creatine via enzymatic assay | Other hemolytic markers included lactate dehydrogenase (LDH), reticulocyte count, haptoglobin; also included blood flow velocity and valvular regurgitation (determined by Doppler echocardiography), and cardiac muscle markers (myoglobin and myosin light chain 1) | Y | 66 | 33 patients with prosthetic valves (15 with aortic valve replacement, 13 with mitral valve replacement, 5 with double-valve replacement); and 33 control subjects | Erythrocyte creatine and LDH levels were significantly higher and haptoglobin level was lower in patients with a prosthetic valve as compared with control subjects; no significant differences in these markers between those with (n = 17) and without (n = 16) regurgitation; patients with high erythrocyte creatine levels (>1.8 umol/g hemoglobin) exhibited significantly higher total peak flow velocity (sum of peak flow velocities at mitral and aortic valves) than those with normal erythrocyte creatine levels; erythrocyte creatine had a significant correlation with total peak flow velocity, but LDH and haptoglobin had no significant correlation with total peak flow velocity; patients with high LDH levels (>460 IU/L) showed significantly higher myoglobin and myosin light chain 1 than those with normal LDH levels, whereas erythrocyte creatine was not related to cardiac muscle markers | Erythrocyte creatine is a quantitative and reliable marker for intravascular hemolysis in patients with prosthetic valves; mild hemolysis is ascribable to valvular flow velocity rather than regurgitation |
| 909 | Diagnostic value of serum transferrin receptor and glycosylated hemoglobin on hemolytic anemia | C.-H. Ho, J.-Y. You, W.-K. Chau, H.-C. Hsu, J.-P. Gau, C.-C. Chen, T.-J. Yu | 2003 | Annals of Hematology | Taiwan | Prospective cohort | – | Soluble serum transferrin receptor (sTfR) and glycosylated hemoglobin (GHb); sTfR was determined by enzyme-linked immunosorbent assay (ELISA) technique, GHb was measured as Hb A1c | Other data included serum ferritin, plasma hemoglobin, complete blood count, reticulocyte, haptoglobin, lactate dehydrogenase, Hb A1c, liver and renal function, direct and indirect bilirubin, fasting blood sugar | Y | 41 | Group A (13 patients with hemolytic anemia with effective erythropoiesis), Group B (13 patients with hemolytic anemia with ineffective erythropoiesis), Group C (15 healthy controls), Group D (summated groups A and B) | Plasma Hb, hematocrit, mean corpuscular volume (MCV), platelet, haptoglobin, LDH, indirect bilirubin, Hb A1c, and sTfR were found to be significantly different between the controls and the hemolytics, either with effective or ineffective erythropoiesis; reticulocyte count was significantly different only between the two hemolytic groups | Hb A1c and sTfR were both good for the diagnosis of hemolysis; reticulocyte count was a good tool for distinguishing effective erythropoiesis (EE) from ineffective erythropoiesis (IE); they could give more information to confirm the presence of hemolysis although they could not define the specific disease of hemolytic anemia |
| 974 | Prediction of hyperbilirubinemia in near-term and term infants | David K. Stevenson, Avroy A. Fanaroff, M. Jeffrey Maisels, Betty W. Y. Young, Ronald J. Wong, Hendrik J. Vreman, James R. MacMahon, Chap Y. Yeung, Daniel S. Seidman, Rena Gale, William Oh, Vinod K. Bhutani, Lois H. Johnson, Michael Kaplan, Cathy Hammerman, Hajime Nakamura | 2001 | Journal of Perinatology | USA | Prospective cohort | Natus Medical, H. M. Lui Research Fund | ETCOc (end tidal carbon monoxide corrected for ambient CO) as a single measurement or in combination with STB (serum total bilirubin); ETCOc measured using CO-Stat end tidal breath analyzer | Coombs' testing | Y | 1370 | Neonates; 120 (8.8%) became hyperbilirubinemic | 120 (8.8%) of the enrolled infants became hyperbilirubinemic; mean STB in breastfed infants was 8.92 ± 4.37 mg/dL at 96 h versus 7.63 ± 3.58 mg/ dl in those fed formula only; the mean ETCOc at 30 ± 6 h for the total population was 1.48 ± 0.49 ppm, whereas those of non-hyperbilirubinemic and hyperbilirubinemic infants were 1.45 ± 0.47 and 1.81 ± 0.59 ppm, respectively; 76% (92 of 120) of hyperbilirubinemic infants had ETCOc greater than the population mean; an ETCOc greater than the population mean at 30 ± 6 hours yielded a 13.0% positive predictive value (PPV) and a 95.8% negative predictive value (NPV) for STB >95th percentile; when infants with STB >95th percentile at <36 h of age were excluded, the STB at 30 ± 6 hours yielded a 16.7% PPV and a 98.1% NPV for STB >75th percentile; the combination of these two measurements at 30 ± 6 h (either ETCOc more than the population mean or STB >75th percentile) had a 6.4% PPV with a 99.0% NPV | This study supports previous observations that measuring STB before discharge may provide some assistance in predicting an infant's risk for developing hyperbilirubinemia; the addition of an ETCOc measurement provides insight into the processes that contribute to the condition but does not materially improve the predictive ability of an hours of age-specific STB in this study population; the combination of STB and ETCOc as early as 30 ± 6 h may identify infants with increased bilirubin production (e.g., hemolysis) or decreased elimination (conjugation defects) as well as infants who require early follow-up after discharge for jaundice or other clinical problems such as late anemia |
| 989 | End-tidal carbon monoxide is predictive for neonatal non-hemolytic hyperbilirubinemia | Hiroko Okuyama, Masahiko Yonetani, Yoshiyuki Uetani, Hajime Nakamura | 2001 | Pediatrics International | Japan | Prospective cohort | – | ETCOc via breath carbon monoxide analyzer (Baby's breath carbon monoxide analyzer) | Hyperbilirubinemia was defined using peak total serum bilirubin concentration, measured via transcutaneous bilirubin index | Y | 51 | Healthy, full-term, non-hemolytic, newborn Japanese infants; seven developed hyperbilirubinemia | ETCOc levels in non-hyperbilirubinemic infants were decreased in the first 72 h after birth; however, those in the hyperbilirubinemic infants were not decreased significantly, and were higher than those in non-hyperbilirubinemic infants at 42, 48, 54 and 66 h of age; ETCOc level at 42 h of age was the most predictive of subsequent hyperbilirubinemia by ROC analysis; at the cut-off level of 1.8 μL/L, the sensitivity, specificity, positive predictive value, and negative predictive value were 86, 80, 40 and, 97%, respectively | Increased ETCOc level in the early neonatal period is associated with subsequent hyperbilirubinemia, even in infants without hemolytic disease; the ETCOc measurement may be useful as a screening test for predicting hyperbilirubinemia without hemolytic diseases; an ETCOc level ≥ 1.8 ppm at 42 h of age is associated with subsequent hyperbilirubinemia, even in infants without hemolytic diseases |
| 1000 | Erythrocyte creatine as a marker of excessive erythrocyte destruction due to hypersplenism in patients with liver cirrhosis | Yu-Fei Jiao, Toshika Okumiya, Toshiji Saibara, Yoshihiro Kudo, Tetsuro Sugiura | 2001 | Clinical Biochemistry | Japan | Prospective cohort | Japan Society for the Promotion of Science, Kurozumi Medical Foundation | Erythrocyte creatine via enzymatic assay; EDTA-treated blood | Other data included spleen size (via ultrasonography, expressed as spleen index), reticulocyte count | Y | 100 | 50 with post necrotic liver cirrhosis, 50 healthy controls | Patients with splenomegaly showed significantly higher erythrocyte creatine than those without splenomegaly and healthy controls, but there was no significant difference in erythrocyte creatine between healthy controls and those without splenomegaly; 14 (93%) of the 15 patients with abnormally high erythrocyte creatine (>1.8 mmol/g hemoglobin) had splenomegaly; there were no significant differences in reticulocyte count between healthy controls and the patients with and without splenomegaly; erythrocyte creatine showed good correlations with spleen index and reticulocytes | Erythrocyte creatine can be used for predicting erythropoietic status and estimating hypersplenism in patients with liver cirrhosis |
| 1033 | Diagnosis of the hemolytic state using serum levels of erythrocyte adenylate kinase | Edward R. Burns, Anjali Kale, Vadaraja V. Murthy | 2000 | American Journal of Hematology | USA | Prospective cohort | – | Erythrocyte adenylate kinase (EAK) via Cobas-Fara analyzer, Helena REP CK electrophoresis, and CK isoenzymes fractionating agarose gels | Serum bilirubin, LDH, reticulocyte count; diagnosis of hemolytic state made by falling hemoglobin, increased reticulocyte count, elevated conventional markers of RBC destruction | Y | 99 | 30 patients in healthy control group, 25 patients in hemolysis group, 23 patients in acute myocardial infarction group, and 21 patients in assorted conditions causing hepatic dysfunction group | The normal range of serum EAK was determined in 30 healthy nonanemic voluntary blood donors and was 0–3.5 Units (mean = 0.5); in 25 patients with hemolytic anemia due to sickle cell disease, hemolytic transfusion reactions, or TTP, the mean EAK level was 62.4 with a range 0–298 units; levels of EAK exceeded the normal range in 24 of 25 patients (96%); in a control group of 44 hospitalized patients with liver disease or myocardial infarction and no clinical evidence of hemolysis, the mean EAK level was 0.12 with a range of 0–3.2; none of the control patients had EAK levels that exceeded the normal range; the diagnostic sensitivity of the EAK assay for hemolysis, as calculated according to Baye's algorithm, was 96%, with a specificity and accuracy of 97%. | Measurement of serum EAK represents a highly sensitive and specific test for the diagnosis of hemolytic anemia; its clinical utility in the diagnosis of anemia would be to differentiate between disorders of red cell destruction and those of inadequate production or blood loss; the sensitivity of the test was no better than the reticulocyte counting this study, but its specificity was superior; reticulocyte count has poor sensitivity for hemolysis in other patient populations whose erythropoietic response is known to be blunted; EAK assay early on in a diagnostic algorithm for anemia diagnosis should serve to reduce the total number of tests performed and speed the determination of the etiology of the anemia |
| 1104 | End tidal carbon monoxide concentration in childhood haemolytic disorders | GCF Chang, YL Lau, CY Yeung | 1998 | Journal of Paediatrics and Child Health | China | Prospective cohort | – | ETCO via one way T-adaptor and anesthetic bag, analyzed by gas chromatography, and carboxyhemoglobin (HbCO) measured for correlation analysis with ETCO | Carboxyhemoglobin, serum indirect bilirubin, fecal and urinary bilirubin, hemoglobin, reticulocyte count | Y | 69 | 31 children with B thalassemia major, 15 with other hemolytic diseases, 23 healthy; age 3–15 years old | Mean ETCO concentrations were 3.21 ppm (B thalassemia major group), 7.41 ppm (other hemolytic disease group) and 0.69 ppm (healthy group), which were significantly different from each other; significant correlation between ETCO and HbCO in the patient groups | End expiratory breath collection device is a simple and feasible sample collection method; results confirm that ETCO can be used clinically to distinguish children with a variety of hemolytic disorders from normal subjects; of all measures for hemolysis, exhaled CO is the most direct and instantaneous measurement; of the methods for determining exhaled CO, ETCO is the simplest method (non-invasive, simple, no blood taking); may also serve as a screening test for differentiating various other conditions associated with anemia or jaundice from hemolytic disorders in children |
| 1151 | Evaluation of a fully automated end-tidal carbon monoxide instrument for breath analysis | Hendrik J. Vreman, Louise M. Baxter, Robert T. Stone, David K. Stevenson | 1996 | Clinical Chemistry | USA | Prospective cohort | National Institute of Child Health and Human Development, Mary L. Johnson Neonatal Research Fund, Christopher Taylor harrison Fund | ETCOc via Baby's Breath Carbon Monoxide Analyzer (Natus Medical) | Gas chromatography, carboxyhemoglobin (COHbc) | Y | 34 | 10 healthy smoking adults, 15 healthy nonsmoking adults, 9 term neonate patients in the neonatal ICU | Bench tests demonstrated that CO measurements were linear, accurate, and precise when compared with gas chromatography (GC) results; in vivo tests (n = 30) performed with adults showed excellent correlation between end-tidal breath CO measurements (ETCO) corrected for inhaled CO (ETCOc) as determined by Baby's Breath and gas chromatography; breath sampling efficiency was 96%; ETCOc measurements and blood carboxyhemoglobin fractions (% of total hemoglobin) corrected for inhaled CO (COHbc) correlated strongly; the imprecision, assessed by the mean of the population's CV for triplicate determinations, was 11%; measurements with healthy and hemolytic term newborns showed that ETCOc values of >3 uL/L correlated with known hemolytic conditions | This instrument is clinically reliable and can be used to noninvasively measure ETCO in neonates and adults; ETCO measurements with the Baby's Breath instrument accurately reflect COHbc concentrations, which in turn reflect the rate of heme degradation and bilirubin destruction; the device is portable, performs fast, fully automated sampling and quantification of CO in breath with good accuracy and acceptable precision for clinical use |
| 1169 | Intravenous immune globulin in neonatal immune hemolytic disease: does it reduce hemolysis? | C. Hammerman, H. J. Vreman, M. Kaplan, D. K. Stevenson | 1996 | Acta Paediatrica | Israel | Prospective cohort | Mirksy Fund, National Institute of Health Grants, Mary L. Johnson Research Fund | Carboxyhemoglobin fraction corrected for inhaled carbon monoxide (COHbc) via gas chromatography and CO analyzer; study focused on the effect of intravenous immune globulin (IVIG) on hemolysis in infants | Other data included serum total bilirubin, total hemoglobin (tHb) | Y | 26 | Term, hyperbilirubinemic, Coombs' positive infants | Babies who responded with a decrease in serum total bilirubin (n = 19), no exchange transfusions were required and COHbc levels decreased significantly by 24 h post-IVIG from 1.37 ± 0.31 to 1.12 ± 0.26% tHb; there were no corresponding decreases in COHbc levels (1.89 ± 0.54 to 1.82 ± 0.48% tHb) among those whose serum bilirubin levels did not decrease in response to IVlG (n = 7), and all of these infants required exchange transfusions; furthermore, the extent of the decrease in COHbc was related to the degree of decrease in serum bilirubin levels, such that the percentage decrease of bilirubin at 24 h was directly correlated with the percentage decrease of COHbc at 24 h | IVIG, when successful, inhibits hemolysis in these infants; concomitant reductions in the responders in both severity of jaundice and degree of hemolysis, as documented by decreased COHbc levels, IVIG action is mediated via inhibition of hemolysis; the fact that there was no decrease in COHbc levels observed in those infants who did not respond further supports the theory that IVIG is effective only when and if it inhibits hemolysis |
| 1206 | Semiportable electrochemical instrument for determining carbon monoxide in breath | Hendrik J. Vreman, David K. Stevenson, William Oh, Avroy A. Fanaroff, Linda L. Wright, James A. Lemons, Elizabeth Wright, Seetha Shankaran, Jon E. Tyson, Sheldon B. Korones, Charles R. Bauer, Barbara J. Stoll, Lu-Ann Papile, Edward F. Donovan, Richard A. Ehrenkranz | 1994 | Clinical Chemistry | USA | Prospective cohort | National Institute of Health, National Institute of Child Health and Human Development | ETCOc in breath via semiportable electrochemical instrument (EC-CO instrument) | Gas chromatography; also recorded serum total bilirubin | Y | 108 | Healthy, 1-day-old infants of non-smoking mothers | CO determination in breath samples compared favorably with determinations by gas chromatography, 1.3 ± 0.8 versus 1.2 ± 0.8 respectively, with a regression equation of EC = 0.95 GC + 0.13; H2 is an interferent that is not trapped by the activated charcoal, the sensitivity of CO to H2 interference ranged from 0% to 52% with a mean SD of 21% ± 14%; hemolytic Coombs' positive neonates (plasma bilirubin > 130 mg/L)had significantly higher CO concentrations (1.8 ± 0.8 uL/L) than healthy Coombs' test negative neonates (1.0 ± 0.7 uL/L) | EC-CO instrument yields results that are comparable with those obtained by the more difficult to perform gas chromatography assay; EC-CO breath instrument allows accurate bedside measurement of CO in small samples of breath and ambient air; measurement of infants' breath within the first 8 h postpartum may allow for the noninvasive diagnosis of hemolysis |
| 1253 | Haptoglobin as a sensitive marker of hemolysis in HELLP-syndrome | G. Wilke, W. Rath, E. Schutz, V.W. Armstrong, W. Kuhn | 1992 | International Journal of Gynecology and Obstetrics | Germany | Prospective cohort | – | Haptoglobin via rate nephelometry (Beckmann Array immunochemistry system) | Other data included plasma bilirubin, LDH, free hemoglobin, peripheral blood smear, platelet count; also, hematocrit, leucocytes, hemostatic variables (PTT, thromboplastin time, fibrinogen, AT-III), AST, ALT, uric acid, creatinine, total protein and plasma electrolytes | Y | 25 | HELLP-syndrome | Reduced haptoglobin levels were observed in all 25 patients at diagnosis; elevated bilirubin and plasma hemoglobin levels were observed in 5/25 patients while an abnormal peripheral blood smear was found in 11/25 patients; as the disease progressed clinically, both haptoglobin levels and thrombocyte counts decreased; the decreases in haptoglobin levels were much more dramatic (−92% of initial value on admission) than the drop in thrombocyte levels (−24%) | Haptoglobin is a sensitive parameter for early detection of moderate hemolysis in HELLP-syndrome; serial measurements of this variable should aid in the accurate diagnosis before it becomes too far advanced and so help reduce maternal and perinatal morbidity and mortality |
| 1316 | Diagnostic value of glycosylated hemoglobin in intravascular hemolysis after cardiac surgery | Chao-Hung Ho, Wing-Keung Chau, Tarng-Jenn Yu, Kwok-Kei Cheng | 1990 | European Journal of Haematology | Taiwan | Prospective cohort | – | Glycosylated hemoglobin (GH) via Glyco-Hemoglobin Kit (Leeco Diagnostics) | Hemoglobin, hematocrit, LDH, serum bilirubin; mentioned other methods such as red cell survival, haptoglobin, LDH, free hemoglobin in plasma, total bilirubin, and reticulocyte count | Y | 42 | Patients with cardiac vascular or valve diseases undergoing cardiac surgery; measurements pre- and post-operatively | In the early post-operation days, Hb and hematocrit, but not GH percentage, were significantly decreased, and LDH significantly increased; this demonstrates that, at this stage, acute blood loss rather than hemolysis is more prominent; in the later post-operative days, GH and total bilirubin, but not Hb, hematocrit or LDH, decreased significantly; this indicates the presence of chronic hemolysis with bone marrow compensation; the incidence of chronic mild hemolysis after cardiac surgery, was very high (68.8%) | GH determination is a simple, easy and sensitive method to detect chronic hemolysis and suggest measuring it in every case with suspicion of hemolysis; simultaneous hemoglobin decrease is not frequent in chronic hemolysis patients, most chronic hemolytic states are “silent” and might be missed if not detected by an adequate technique such as GH determination |
| 1342 | Reduction of neonatal blood eliminates the error induced by oxygen saturation in the spectrophotometric measurement of carboxyhaemoglobin | N. A. Nicholson, C. D. Karabus, M. Klein, P. S. Hartley | 1988 | Annals of Clinical Biochemistry | South Africa | Prospective cohort | – | COHb via IL-282 CO-oximeter (spectrophotometric method); the hemoglobin in neonatal blood was reduced with sodium dithionite to eliminate the problem of fetal hemoglobin (HbF) interfering with COHb measurement | Hopcalite CO analyzers, gas chromatography, Van Slyke manometric method, infrared CO analysis | Y | 93 | 50 babies of non-smoking mothers (uncomplicated deliveries, no evidence of blood group incompatibility), 20 healthy non-icteric neonates, 23 healthy non-smoking adults | COHb levels fell after reduction; significant positive correlation between apparent COHb% and oxygenation of cord blood; in contrast, no significant correlation between these parameters in adult blood where COHb values remained the same or rose slightly after reduction; in 20 healthy neonates, the mean reduced blood COHb value was not significantly different from the mean COHb value of 23 healthy adults | COHb in neonatal blood can be simply and accurately measured by the IL-282 co-oximeter provided the blood is fully reduced; COHb can be measured in neonatal blood by instruments that have been calibrated with adult values provided that the O2Hb saturation is reduced to less than 1.5% (useful for comparing rates of hemolysis in infants with various forms of neonatal jaundice) |
| 1349 | What is the best predictor of the severity of ABO-haemolytic disease of the newborn? | H. A. A. Brouwers, M. A. M. Overbeeke, I. van Ertbruggen, W. Schaasberg, G. P. J. Alsbach, C. van der Heiden, E. F. van Leeuwen, J. W. Stoop, C. P. Engelfriet | 1988 | The Lancet | Netherlands | Prospective cohort | – | Serum bilirubin, reticulocytes, spherocytes, hemoglobin concentration, LDH and its isoenzymes, glutathione reductase; all to seek signs of hemolytic disease | Other investigations included the titre of IgG anti-A or anti-B antibodies via ADCC (antibody-dependent cell-mediated cytotoxicity assay), indirect antiglobulin test, direct antiglobulin test, antigen density via ELISA | Y | 200 | 80 newborn infants with ABO-incompatibility with their mothers; 120 ABO-compatible infants | There were significant differences between group 1 (ABO-incompatible, predicted to be affected, ADCC positive, high antigen density) and group 2 (ABO-incompatible, predicted to be unaffected, ADCC negative, low antigen density) for the hourly rise in serum bilirubin on Day 1, the hourly rise until the maximum bilirubin concentration was reached, the mean serum bilirubin on Day 2, the mean maximum serum bilirubin, the mean hemoglobin concentration on Days 2 and 7, the mean fall in hemoglobin between Day 2 and Week 4, the reticulocyte count on Day 2, the titre of IgG anti-A or anti-B antibodies in cord blood, the titre of maternal anti-A or anti-B antibodies (total IgG and each of the four subclasses), and the number of children with a positive direct antiglobulin test on the cord blood red cells; there was no significant difference between Groups 1 and 2 for the spherocyte count or serum levels of lactate dehydrogenase, its isoenzymes, and glutathione reductase; no association between the antigen density on cord-blood red cells or the titre of cord-blood IgG anti-A or anti-B antibodies and a positive direct antiglobulin test; no correlation was found between the antigen density on cord-blood red cells and gestational age nor between the number of pregnancies and the result of the ADCC, the titre of maternal IgG anti-A or anti-B antibodies, or the subclasses of the maternal IgG anti-A or anti-B antibodies | The ADCC (antibody-dependent cell-mediated cytotoxicity) assay with maternal serum was the most sensitive assay to predict ABO-HDN, and the combination of the ADCC assay with A or B antigen density determination the most specific test; all hematological features are indirect effects of hemolysis, and the serological features poorly predict the degree of hemolysis or the need for therapy in individual cases; no significant difference in the spherocyte count between Groups 1 and 2 is unexpected, because the number of spherocytes is usually high in patients in whom Fcy-receptor-mediated destruction of red cells occurs; the lytic effect of maternal antibodies in an ADCC assay, alone or combined with the antigen density on the red cells of the infant, is a good predictor of hemolysis and the need for therapy in ABO-incompatible infants; lack of correlation between the titre of maternal IgG anti-A or anti-B antibodies or the outcome of the direct antiglobulin test and the degree of hemolysis in many cases |
| 1426 | Neonatal bilirubin production estimated from “end-tidal” carbon monoxide concentration | David W. Smith, Andrew O. Hopper, Susan M. Shahin, Ronald S. Cohen, Clinton R. Ostrander, Ronald L. Ariagno, David K. Stevenson | 1984 | Journal of Pediatric Gastroenterology and Nutrition | USA | Prospective cohort | General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health, Thrasher Research Fund, Mead Johnson Nutritional Division | ETCO (approximate end tidal sample of CO via gas chromatography), VECO (pulmonary excretion rate of CO) | COHb, Coombs test, reticulocytosis; described that ETCO estimates VECO which estimates bilirubin | Y | 23 | 9 preterm infants, 14 term infants | VECO and ETCO correlated significantly over a wide range of CO elimination rates; ETCO correctly predicted elevations in VECO >2 SD of the mean VECO for normal infants with 90% sensitivity and 73% specificity; 3 subjects with Rh isoimmune hemolytic disease were easily identified by ETCO as well as VECO | ETCO is a simple, noninvasive measurement for rapidly identifying infants with significant hemolytic disease; in infants without pulmonary disease, it is possible to obtain a correlation of ETCO with VECO, an accepted index of total bilirubin formation; neither the degree of positivity of a Coombs test nor the degree of reticulocytosis is necessarily correlatable with the rate of hemolysis; a more direct measure of hemolytic rate is provided by an estimation of the rate of bilirubin production, accurately reflected by the VECO or ETCO; ETCO is faster and simpler than VECO testing |
| 1521 | Measurement of haemolysis in patients with prosthetic heart valves | J. A. F. Napier, I. Cavill, C. Ricketts | 1979 | Cardiovascular Research | Wales/UK | Prospective cohort | – | 59-Fe ferrokinetic technique (Cavill technique); 51-Cr-labeled red cells was also measured | Serum hydroxybutyrate, reticulocyte count, serum haptoglobin, urinary hemosiderin, RBC fragmentation; LDH, 24 h urinary iron loss | Y | 10 | Patients who had an aortic prosthetic heart valve inserted | Mean red blood cell lifespan in these patients ranged from 27 to 98 days and was not related to the presence of anemia; significant correlation between the 51-Cr survival and 59-Fe red cell lifespan; in six subjects (60%) with hemoglobin concentrations within the normal range there was shortening of the red cell lifespan; in three of these the red blood cell lifespan was markedly shortened though reticulocyte counts had not shown significant elevation; hemolysis was also demonstrated in the two anemic patients, one of these showing elevated serum hydroxybutyrate dehydrogenase and reticulocyte count; serum haptoglobin levels were generally subnormal and urinary hemosiderin noted in all except one patient; likewise, the presence of red blood cell fragmentation did not correlate with the presence of hemolysis | The precise determination of the degree of hemolysis associated with prosthetic heart valves can only be accomplished by direct measurement of red cell lifespan; this may involve the use of an extrinsic red cell label such as 51-Cr but this technique may be particularly susceptible to errors in patients with mechanical hemolysis; use of 59-Fe as a tracer which is physiologically incorporated into red cells has a number of advantages over extrinsic labels which may make this technique particularly suitable for the assessment of hemolysis in a variety of circumstances where direct estimates of slight disturbances of red cell lifespan in potentially hemolysing patients are required; the use of 59-Fe labeled transferrin provides estimates of total and ineffective erythropoiesis |
| 1533 | Comparison of red cell creatine level and reticulocyte count in appraising the severity of hemolytic processes | Jorg Fehr, Margarethe Knob | 1979 | Blood | Switzerland | Prospective cohort | – | Erythrocyte creatine via 51-Cr-labeling | Reticulocyte count, level of oxygen-dissociation regulator 2,3-DPG | Y | 61 | 21 patients with steady-state hemolysis/overt hemolytic disease, 40 healthy patients | High correlation between reticulocyte counts and red cell creatine levels; excluding elevation of the creatine level as a variable epiphenomenon of increased erythropoietic activity, density separation of normal red cells revealed distinctly higher creatine levels in younger cells; the reticulocyte counts and creatine levels as quantitative predictors of hemolytic processes were compared: in severe hemolytic anemias, erythrocyte survival correlated well with creatine levels and to a lesser degree with reticulocyte counts; in milder disease, no correlation existed between reticulocyte counts and erythrocyte survival whereas the creatine levels correlated closely with erythrocyte survival; no significant correlation between RBC creatine and 2,3-DPG; 3/21 hemolytic patients had normal creatine values, whereas 11/21 hemolytic patients had normal reticulocyte counts | Useful estimation of red cell survival may be obtained from single measurements of erythrocyte creatine; creatine measurements allow better estimation of degree of RBC destruction than can be deduced from a so-called reticulocyte production index; creatine is the best currently available index to check for steady-state conditions during any studies involving red cell kinetics |
- Note: All studies were completed. Abbreviations are explained in Table 1.
Open Research
DATA AVAILABILITY STATEMENT
All data are provided in the main text and appendices.




