Efficacy of denosumab monotherapy among adults with Langerhans cell histiocytosis: A prospective clinical trial
Graphical Abstract
This phase IIb clinical trial evaluated the efficacy of a bimonthly treatment schedule (Q8W) with 4 subcutaneous doses of denosumab 120 mg among adults with Langerhans cell histiocytosis needing first-line systemic therapy for either multifocal single-system disease or multisystem disease without risk organ involvement. Two months after the last treatment administration, seven patients showed disease regression, one stable disease, one non-active disease, and one disease progression. One year after treatment, progression was evident in two patients, while the remaining exhibited either a regression (three patients) or non-active disease (five patients). No permanent sequalae developed during the study and no adverse events were adjudicated in treatment. In conclusion, four doses of denosumab 120 mg Q8W subcutaneously are an effective treatment option in Langerhans cell histiocytosis patients without risk organ involvement exhibiting a response rate of 80%. Further studies are needed to confirm its role as a disease modifying agent.
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia characterized by clonal proliferation of abnormal myeloid precursor cells induced by oncogenic mutations of the MAPK/ERK pathway and the recruitment and activation of inflammatory cells.1 We have previously shown that receptor activator of NF-kB ligand (RANKL) is abundantly expressed in cells within diverse LCH lesions in adult patients,2 in line with the previously reported high osteoprotegerin (OPG) and low RANKL levels in the serum of patients with LCH. These findings support our initial hypothesis of a swift of circulating RANKL to LCH-related tissue lesions that induces a compensatory rise of serum OPG.2 It can thus be inferred that a compensatory OPG production against lesional RANKL is an intrinsic disease-related ongoing process supporting the use of an anti-RANKL agent such as denosumab as a rational treatment for patients with LCH. In a previous report we demonstrated that a bimonthly treatment schedule with four doses of denosumab 120 mg subcutaneously (SC) resulted in disease regression at both skeletal and non-skeletal sites.3 To further explore that, we designed this prospective, single-arm, open-label, multicenter, efficacy study aiming to assess the efficacy of this denosumab regimen in adult LCH patients with mild symptoms and low-risk disease requiring first-line systemic therapy.
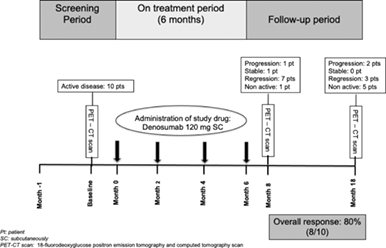
“Denosumab for the Treatment of Adult LCH” was an 18-month interventional study (https://clinicaltrials.gov/ct2/show/NCT03270020; EudraCT number 2016-003300-31). Adult patients with a histologically proven definitive diagnosis of LCH and active disease indicated by a positive [18F] fluorodeoxyglucose (18F-FDG) positron emission tomography and computed tomography (PET/CT) scan were eligible to participate if they were exhibiting mild symptoms (symptoms of low intensity; no need for hospitalization) and low-risk disease requiring first-line systemic therapy for LCH due to the following: single-system disease with multifocal lesions or single-system disease with “special site” lesions (vertebral lesions with intraspinal extension, craniofacial bone lesions with soft tissue extension); multisystem disease without risk organ involvement (RO−) (hematopoietic system, spleen, liver, or tumorous central nervous system [CNS]). All patients received four doses of denosumab 120 mg every 8 weeks (Q8W) SC and were followed for 12 more months. The study protocol included 10 visits for each patient (Supplementary Figure 1). Throughout the study, all patients were given cholecalciferol 800 IU/day and calcium carbonate (500 mg b.i.d.) supplements and a monthly dose of cholecalciferol 25 000 IU to avoid hypocalcemic events. Symptomatic multisystem LCH requiring hospitalization, multisystem LCH with risk organ(s) involvement, or isolated pulmonary LCH were the major exclusion criteria, while the list of the remaining is provided in the supplemental material.
A formal hypothesis was not tested; however, the proportion of subjects with progressive disease at two different time points (2 months after last treatment administration—end of study) was evaluated. This was an investigator sponsored study of the Hellenic Society for the Study of Bone Metabolism (HSSBM), performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
The International LCH Study Group criteria4 were modified to include the radiographic response according to the PET/CT findings and were used to evaluate the response to treatment. Patients were categorized as having either non-active disease or active disease at a stable, regressive, or progressive stage (Supplementary Table 1). Specifically, regarding the study endpoints, the activity of the disease was assessed through clinical examination, early-morning pre-specified laboratory blood and urine examinations, radiological examinations (e.g., ultrasound, high-resolution CT) depending on each individual's organ involvement, and 18F-FDG PET/CT scan. Qualitative assessment of the response to treatment was performed through direct comparison of PET/CT studies performed at baseline, 2-months after the last denosumab administration (8 months), and at the end of the study (18 months).
The primary efficacy endpoint of the study was the number of patients with active disease 2 months after last Denosumab administration; secondary efficacy endpoint was the incidence of disease-related permanent sequelae developed during the study, namely diabetes insipidus (DI), anterior pituitary deficiencies (APDs), and pulmonary failure; exploratory endpoint was the number of patients with active disease at the end of study; safety endpoint was the incidence of all adverse events (AEs) during the 18-month trial period.
Ten adult patients (five females and five males), with a mean age of 38.5 ± 15.05 years (range: 20–57 years), were finally enrolled and completed the 18-months follow up according to the protocol. At baseline, one patient had single-system disease with multifocal lesions and nine patients had multisystem RO− disease.
Progression of the disease at month 8 (primary efficacy point) was documented in only one patient (patient No 1) who developed a new bone lesion with regression of all initial lesions (Table 1). Of the remaining nine patients, one still had active but stable disease, seven had regression of the disease, while in one patient, no disease activity was detected. Thus, the response rate was calculated to be 80%, along with 10% stable disease.
| Pt no | Gender | Age | Endocrine deficienciesa | Extent of active disease at baseline based on PET/CT | Symptoms and signs at baseline | Disease status at 8 monthsb | Disease status at end of study | Definition of disease status at end of study |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 20 | None | Multiple bone lesions (R femur, L pubic bone), paratracheal lymph node | Skeletal pain | Active progressive | Non-active | Resolution of all PET/CT findings and symptoms |
| 2 | F | 42 | DI | Multiple bone lesions (maxilla, both tibiae, metatarsals, heels, talar bones), small intestine, jugular lymph nodes | Skeletal pain | Active stable | Active regression | Regression of PET/CT findings, no symptoms, no new lesions |
| 3 | F | 57 | None | Inguinal lymph nodes, bone lesion (rib) | Painful lymph node enlargement, skeletal pain | Active regression | Active regression | Regression of PET/CT findings, no symptoms, no new lesions |
| 4 | M | 57 | None | Bone lesion (rib), lungs, paratracheal lymph node | Skeletal pain | Non-active | Non-active | Resolution of all PET/CT findings and symptoms |
| 5 | F | 50 | DI, GH, FSH/LH | Soft tissue masses of right hip, lungs | Lesional pain and discomfort | Active regression | Active progressive | Progression of radiological signs (including PET/CT) of soft tissue involvement, no symptoms, new soft tissue lesion |
| 6 | F | 21 | DI | Multiple cervical and thoracic soft tissue masses, skin, mediastinal lymph nodes | Skin erosion, lesional pain and discomfort | Active regression | Non-active | Resolution of all PET/CT findings and symptoms |
| 7 | F | 50 | DI, GH | Single bone lesion (L femur), cervical lymph nodes, lungs | Lymph node enlargement, skeletal pain | Active regression | Active regression | Regression of PET/CT findings, no symptoms, no new lesions |
| 8 | M | 40 | None | Single bone lesion (R shoulder blade), lungs | Skeletal and radiating muscular pain | Active regression | Non-active | Resolution of all PET/CT findings and symptoms |
| 9 | M | 22 | None | Multiple bone lesions (R femur, L ilium, L5 vertebra) | Skeletal pain | Active regression | Non-active | Resolution of all PET/CT findings and symptoms |
| 10 | M | 26 | None | Multiple bone lesions (both shoulder blades, mandible), lungs | Skeletal pain | Active regression | Active progressive | Regression of initial PET/CT findings, no symptoms, new bone lesion |
- Abbreviations: DI, diabetes insipidus; F, female; FSH/LH, follicular stimulating hormone/luteinizing hormone (central hypogonadism); GH, growth hormone; L, Left; M, male; PET/CT, [18F] fluorodeoxyglucose positron emission tomography and computed tomography scan; Pt No, patient number; R, right.
- a At baseline.
- b Last denosumab administration took place at 6 months.
None of the patients without pituitary involvement at baseline developed either DI or APD throughout the 18 months of the study. Moreover, none of the four female patients with pituitary involvement developed additional APDs during follow-up and daily requirements of desmopressin remained unchanged among the four patients with DI.
Lung involvement was evident in PET/CT in five patients at baseline, all of them smokers (Table 1). None of the patients without lung involvement at baseline developed lung lesions throughout the study period. According to the protocol the five patients with lung involvement were followed with lung function tests at four study visits (Supplementary Tables 2–5). Despite strong guidance to quit smoking, they only reduced cigarette consumption. No significant difference was noted among the lung function parameters throughout the study, suggesting no deterioration of lung function in these patients (Supplementary Table 6).
At end of the study (18 months) patient No 1, who showed progression at the visit of the primary endpoint (8 months), had non-active disease (Table 1). Among the remaining nine patients, progression of the disease was evident in two of them (20% of total), while the remainder of the patients exhibited either a regression of their active disease (three) or non-active disease (five) (Table 1; Supplementary Figure 2). Overall, this corresponds to an 80% response rate at the end of study. Although this was not included in the study design, we can also confirm that none of the patients with either regression or non-active disease required additional treatment during an additional off-study follow-up of 22.1 ± 4.5 months (range: 3–42 months).
AEs were recorded during the pre-specified visits and when spontaneously reported by the study subjects, while specific attention was given in case of a reasonable possibility that the study treatment caused the event. The severity of AEs was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 of the US National Cancer Institute. No serious AEs were reported during the study and none of the patients discontinued the study. Only three grade 1 AEs were recorded with a potential relationship to the study treatment; two patients were diagnosed with upper respiratory tract infection and one patient with gastroenteritis at some point during the study. However, none of these AEs was adjudicated to the study drug/treatment. No cases of osteonecrosis of the jaw were observed during the study. No hypocalcemic events were noted throughout the study and no patient has experienced any skeletal event attributed to denosumab discontinuation.
The fluctuation of blood and urine laboratory parameters were within the expected values throughout the study. Erythrocyte sedimentation rate and CRP levels exhibited a continuous decreasing trend that failed marginally to reach statistical significance (Supplementary Table 7).
In this study, we found that four doses of denosumab 120 mg every 8 weeks (Q8W) SC provided an overall response rate of 80% among low-risk adult patients with mild symptoms, thus indicating a beneficial effect of denosumab at all studied sites of LCH involvement. Current treatment recommendations for symptomatic adult multisystem or multifocal single-system LCH RO− include chemotherapy as the first-line approach.1 The exceptions to this chemotherapy-based approach are bone-only disease where bisphosphonates with or without oral methotrexate and hydroxyurea could be initially chosen, and skin-only disease in which topical therapy, methotrexate, 6-mercaptopourine, or immunomodulatory imide drugs may be used as first-line management.1, 5
As no randomized controlled clinical trial for the treatment of adult LCH currently exists, according to the protocol, the results from this study were considered relative to response rates with standard treatment from previously reported studies and case series. In general, treatments that have been used among adult LCH patients are either vinca alkaloid (vinblastine, vincristine)/steroid-based or antimetabolite-based (cladribine, cytarabine) regimens. Considering the therapeutic outcomes from historical controls, our therapeutic protocol resulted in a high response rate (80%) which is numerically comparable with the reported outcomes of cladribine and vinca alkaloid/steroid-based regimens series.1 Additionally, the relapse rate 1 year following the last treatment administration was 20%, which is comparable with the around 30% progression rate of the methotrexate-cytarabine regimen, although over the longer period of 3 years.6 Unfortunately, beside the numerical comparisons in response and relapse rates, we cannot directly compare this study with the previous ones as our cohort included low-risk patients in contrast with other studies that mostly involved high-risk patients. The two main limitations of our study are the relatively small number of participants and the lack of an active treatment control group which would directly allow a comparison with other existing therapeutic options.
In conclusion, a course of four doses of denosumab 120 mg every 8 weeks (Q8W) SC constitutes an effective and safe treatment option for low-risk adult patients with LCH with mild symptoms, with a sustainable outcome for at least 1 year. Larger prospective trials with additional treatment arms and a longer period of follow-up are required both among low- and high-risk patients to place denosumab in the therapeutic algorithm.
AUTHOR CONTRIBUTIONS
Polyzois Makras contributed to the study conception and design. Data collection was performed by Polyzois Makras, Maria P. Yavropoulou, and Danai Georgakopoulou, and analysis was performed by Polyzois Makras and Maria P. Yavropoulou. Polyzois Makras, Maria P. Yavropoulou, Sofia N. Chatziioannou, Athanasios D. Anastasilakis, Danai Georgakopoulou, Marina Tsoli, Eleftherios Chatzelis, and Gregory Kaltsas followed and/or evaluated the patients. Polyzois Makras, Maria P. Yavropoulou, Athanasios D. Anastasilakis, and Gregory Kaltsas drafted the manuscript and all authors commented on different versions of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful to Prof. Socrates Papapoulos (Leiden University Medical Center, The Netherlands) for his constructive criticism, ideas, and overall support, starting with the concept of this project and continuing until its completion.
FUNDING INFORMATION
The study was supported by Amgen.
CONFLICT OF INTEREST STATEMENT
Polyzois Makras: lecture fees/advisory boards from Amgen, ELPEN, Farmaserv, Galenica, Genesis, Lilly, Pfizer, Rapharm, Takeda, UCB Pharma, UniPharma, Bennett, and VIANEX; research grants from Amgen, Galenica, ITF. Athanasios D. Anastasilakis: lecture fees from Amgen, Bianex, Eli-Lilly, Galenica, ITF, Unifarma and UCB; consultation fees from Amgen. Gregory Kaltsas: consultation fee from IPSEN. Maria P. Yavropoulou, Sofia N. Chatziioannou, Danai Georgakopoulou, Marina Tsoli, and Eleftherios Chatzelis have nothing to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date. Requests for access should be sent to [email protected]. The analytical study protocol is immediately available upon request to [email protected].