Predictors of impaired antibody response after SARS-CoV-2 mRNA vaccination in hematopoietic cell transplant recipients: A Japanese multicenter observational study
Abstract
HCT recipients reportedly have a high mortality rate after developing COVID-19. SARS-CoV-2 vaccination is generally useful to prevent COVID-19. However, its safety and efficacy among HCT recipients remain elusive. This large-scale prospective observational study including 543 HCT recipients with 37-months interval from transplant demonstrated high safety profiles of mRNA vaccine: only 0.9% of patients avoided the second dose due to adverse event or GVHD aggravation following the first dose. Regarding the efficacy, serological response with a clinically relevant titer (≥250 BAU/mL) was obtained in 397 (73.1%) patients. We classified the remaining 146 patients as impaired responders and compared the clinical and immunological parameters between two groups. In allogeneic HCT recipients, multivariable analysis revealed the risk factors for impaired serological response as follows: age (≥60, 1 points), HLA-mismatched donor (1 points), use of systemic steroids (1 points), absolute lymphocyte counts (<1000/μL, 1 points), absolute B-cell counts (<100/μL, 1 points), and serum IgG level (<500 mg/dL, 2 points). Notably, the incidence of impaired serological response increased along with the risk scores: patients with 0, 1–3, and 4–7 points were 3.9%, 21.8%, and 74.6%, respectively. In autologous HCT recipients, a shorter interval from transplant to vaccination was the only risk factor for impaired serological response. Our findings indicate that two doses of SARS-CoV-2 vaccine are safe but insufficient for a part of HCT recipients with higher risk scores. To improve this situation, we should consider additional treatment options, including booster vaccination and prophylactic neutralizing antibodies during the SARS-CoV-2 pandemic.
1 INTRODUCTION
The prognosis of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is poor in patients with hematological malignancies and/or patients who received hematopoietic cell transplantation (HCT).1-4 Vaccination has been the most effective option in preventing disease: several pivotal trials demonstrated the safety and efficacy of vaccines in the healthy population.5-7
However, emerging reports from cohort studies have indicated poor humoral responses to SARS-CoV-2 mRNA vaccines in patients with hematologic diseases, especially in patients treated with anti-CD20 antibody, Bruton tyrosine kinases inhibitors, or chimeric antigen receptor (CAR)-T cell therapy.8, 9 With limited data from HCT recipients, various baseline characteristics and immune parameters have been identified as risk factors for inadequate acquisition of antibodies after vaccination.10-17 Guidelines for SARS-CoV-2 vaccination of HCT recipients have been established by the American Society of Hematology and the American Society for Transplantation and Cellular Therapy,18 the European Society for Blood and Marrow Transplantation,19 and the Societe Francophone de Greffe de Moelle et de Therapie Cellulaire20 that were relevant for patients as well as transplant physicians.
Although guidelines are available for the US and Europe, data are still lacking on the safety and immunogenicity/efficacy of SARS-CoV-2 mRNA vaccines in Japanese HCT recipients. To date, only a few single-center analyses with small numbers of patients have been conducted.21, 22 Thus, no guidelines for Japanese patients are available. Therefore, we conducted a multicenter prospective observational study to assess the safety and serological response after SARS-CoV-2 vaccination in 543 Japanese HCT recipients.
2 METHODS
2.1 Patients and eligibility
A total of 595 patients were enrolled in this study between April and December 2021. Written informed consent was obtained from all study participants. The institutional review board at Kurume University Hospital approved the study (UMIN registration number; 000043671), which was conducted by the Fukuoka Blood and Marrow Transplantation Group.
The inclusion criteria for this study were as follows: aged 16 years and over; diagnosis of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL), myelodysplastic syndrome (MDS), aplastic anemia (AA), or malignant lymphoma (ML) including adult T-cell leukemia/lymphoma (ATLL); ≥6 months after the most recent HCT; available data regarding quantitative anti-spike antibody titer before the first dose and 7–49 days after the second dose of the SARS-CoV-2 mRNA vaccine (BNT162b2 [Pfizer/BioNTech] or mRNA-1273 [Moderna]). Patients were excluded from the study if they had (i) grade III to IV acute graft-versus-host disease (aGVHD), (ii) a history of B-cell depleted therapy (i.e., anti-CD20 antibody, CAR-T cell) in the last 6 months, (iii) anti-thymocyte globulin (ATG) or alemtuzumab administration in the last 6 months, or (iv) other serious medical or psychiatric conditions making the patient unsuitable for study entry at the time of registration.
2.2 Endpoints of the study
The primary endpoint of the study was the immunogenicity of the SARS-CoV-2 vaccine, which was measured by the concentration of anti-SARS-CoV-2 receptor binding domain of spike protein subunit 1-conjugated IgG antibody (U/mL) using a double antigen sandwich assay (Elecsys® Anti-SARS-CoV-2 S[RUO]; Diagnostics Roche) between 7–49 days after the second dose of SARS-CoV-2 vaccine. Based on a direct comparison of the values for each assay, the U/mL is equivalent to the bound antibody unit (BAU)/mL.23 Hereafter, we used the term BAU/mL that was more familiar to most transplant physicians instead of U/mL. Secondary endpoints included the correlation between the acquired antibody titer and clinical parameters, blood tests results, and safety profiles.
2.3 Data collection
Clinical data were collected from medical records at each transplantation center, the Transplant Registry Unified Management Program (TRUMP), the database of the Japanese Society for Transplantation and Cellular Therapy,24 or a questionnaire prepared for this analysis. The absolute number of lymphocyte subsets before the first dose of vaccination was evaluated by flow cytometry.
2.4 Statistics
Categorical variables are expressed as n (%), and continuous variables are expressed as the median (range). Possible risk factors for impaired responses were assessed via logistic regression models. Variables with p < 0.15 in the univariable logistic analyses were used as independent variables in the multivariable logistic regression model. A two -sided p < 0.05 was considered statistically significant in the multivariable logistic model. Statistical analyses were performed using JMP PRO ver.15.0 or SAS ver.9.4 (SAS Institute Inc., Cary, NC, USA).
3 RESULTS
3.1 Participants
As indicated in Figure S1, 595 patients from 23 participating institutions were enrolled in this study. Forty-three (7.2%) patients were excluded before the first dose of vaccine due to underlying disease unfit for inclusion criteria (n = 4), positive results of anti-SARS-CoV-2 IgG before vaccination (n = 14), withdrawal of consent (n = 5), disease relapse/recurrence (n = 7), post-transplant complications (n = 6), inappropriate sample collection (n = 3), or unknown reasons (n = 4). In addition, 6 (1.0%) patients did not receive the second vaccine due to GVHD aggravation (n = 4), progressive underlying disease (n = 1), or an adverse event (AE) (n = 1) after the first dose. Anti-SARS-CoV-2 IgG titers were missing in 3 cases. The remaining 543 (91.3%) patients were included in the analysis.
Patient characteristics are summarized in Table S1. The proportion of male patients was 53.8%. Patients with AML or ALL in remission, MDS without excess of blasts, CML in the non-leukemic phase, ML in at least partial remission, and nonmalignant diseases were considered to have standard-risk underlying diseases (n = 348, 64.1%). All other patients, except 7 cases with missing data, were considered to have high-risk underlying diseases (n = 187, 34.4%).
3.2 Summary of recent HCT
As shown in Table S1, 510 (93.9%) patients underwent allogeneic HCT (allo-HCT) and 33 (6.1%) patients underwent autologous HCT (auto-HCT). Forty (7.4%) patients had a prior history of HCT (auto-HCT: 18, allo-HCT: 22). For patients who underwent allo-HCT (including 7 cases with partial deficit of transplant data), the stem cell sources included bone marrow (n = 169, 33.1%), G-CSF-mobilized peripheral blood (n = 146, 28.6%), and cord blood (CB) (n = 195, 38.2%). Stem cells were obtained from HLA fully matched related donors (n = 74, 14.5%); partially matched related donors (n = 76, 14.9%), including 65 (12.7%) haploidentical donors; fully matched unrelated donors (n = 96, 18.8%); and mismatched unrelated donors (n = 65, 12.7%). Myeloablative conditioning and reduced-intensity conditioning regimens were adopted for 330 (64.7%) and 173 (33.9%) patients, respectively. Calcineurin inhibitors (CIs) served as the backbone for the GVHD prophylaxis and were combined with short-term methotrexate (n = 278, 54.5%), mycophenolate mofetil (n = 184, 36.1%), and methylprednisolone (n = 13, 2.5%). Low-dose rabbit ATG (n = 46, 9.0%) or post-transplant cyclophosphamide (PTCy) (n = 56, 11.0%) were administered preferentially to recipients using haploidentical donors. Prior acute GVHD (grade II to IV) was found in 234 (45.9%) patients.
G-CSF-mobilized peripheral blood was used as the stem cell source for 33 auto-HCT recipients. Prior rituximab treatment was administered in 23 (69.7%) patients.
3.3 Clinical and laboratory parameters of HCT recipients at the time of study enrollment
Clinical and laboratory characteristics based on the patients' category (allo-HCT and auto-HCT) are summarized in Table 1. The median age at the time of study enrollment was 53 years (range, 17–77). The interval from the most recent transplantation to vaccination was 40 months (range, 6–287 months) in allo-HCT recipients and 27 months (range, 7–210 months) in auto-HCT recipients; 73 patients (13.4%) underwent vaccination within 12 months of transplantation. In the allo-HCT recipients, 339 (66.5%) patients had no sign of chronic GVHD (cGVHD), whereas 108 (21.2%) patients had limited cGVHD, and 63 (12.4%) patients had extensive cGVHD. Systemic steroid and other immunosuppressant (mainly CIs) treatments were ongoing in 148 (29.0%) and 155 (30.4%) patients, respectively (Table 1).
| Allo-HCT | Auto-HCT | |
|---|---|---|
| (n = 510) | (n = 33) | |
| Clinical parameters | ||
| Age, median (range) | 52 (17–77) | 60 (28–77) |
| ≥60 years, n (%) | 165 (32.4) | 17 (51.5) |
| Interval from the most recent HCT to vaccination | ||
| Months, median (range) | 40 (6–287) | 27 (7–210) |
| <12 m, n (%) | 64 (12.5) | 9 (27.3) |
| cGVHD in allo-HCT recipients | ||
| None, n (%) | 339 (66.5) | - |
| Limited, n (%) | 108 (21.2) | - |
| Extensive, n (%) | 63 (12.4) | - |
| Concurrent immunosuppression | ||
| Systemic steroids, n (%) | 148 (29.0) | - |
| Other ISTs, n (%) | 155 (30.4) | - |
| Immunological parameters | ||
| White blood cell count (/μL), median (range) | 6300 (1700–30 400) | 4800 (1500–10 100) |
| Absolute lymphocyte count (/μL), median (range) | 1950 (307–23 104) | 1624 (331–3965) |
| <1000/μL, n (%) | 53 (10.4) | 2 (6.1) |
| Number of CD4+ T cells (/μL), median (range) | 654.5 (94–5259) | 403 (64–1178) |
| <300/μL, n (%) | 57 (11.2) | 8 (24.2) |
| Number of CD8+ T cells (/μL), median (range) | 819 (95–14 610) | 903 (247–2513) |
| <300/μL, n (%) | 32 (6.3) | 1 (3.0) |
| Number of B cells (/μL), median (range) | 144.5 (4–3279) | 39 (3–648) |
| <100/μL, n (%) | 195 (38.2) | 23 (69.7) |
| Serum IgG level (mg/dL), median (range) | 984.5 (190–2232) | 854 (232–1562) |
| <500 mg/dL, n (%) | 31 (6.1) | 7 (21.2) |
- Abbreviations: cGVHD, chronic graft-versus-host disease; HCT, hematopoietic cell transplantation; IST, immunosuppressants.
Overall, the median white blood cell count was 6200/μL (range, 1500–30 400/μL). The median absolute lymphocyte count (ALC) was 1914/μL (range, 307–23 104/μL), including 641/μL (range, 64–5259/μL) CD4+ T-cells, 827/μL (range, 95–14 610/μL) CD8+ T-cells, and 137/μL (range, 3–3279/μL) B-cells. The median level of serum IgG was 978 mg/dL (range, 190–2232 mg/dL).
3.4 Serological response for two doses of SARS-CoV-2 vaccine
We analyzed the serological response as the primary endpoint. Both vaccines (91.9% with Pfizer and 8.1% with Moderna) were administered in two doses 3–4 weeks apart. After the second dose, antibody response was detectable in 90.4% (491/543) of patients, with a median antibody level of 1190 BAU/mL (range, 0.87–29 800 BAU/mL). No seroconversion (<0.8 BAU/mL) was observed in 52 (9.6%) patients. Antibody titers ≥250 BAU/mL associated with a high (~90%) efficacy25 were obtained in 397 (73.1%) patients, whereas the antibody titers in the remaining 94 (17.3%) patients were <250 BAU/mL. We classified 146 patients (26.9%) with negative to low serological response (<250 BAU/mL) as impaired responders, as previously reported.10
3.5 Univariable and multivariable analyses for impaired serological response among Allo-HCT recipients
The risk factors for inadequate anti-SARS-CoV-2 IgG titer were determined by comparing impaired responders (n = 146) to responders (n = 397). In allo-HCT recipients, the rate of impaired serological response was 25.9%. As indicated in Table 2, multiple parameters showed statistically significant or marginal correlation with impaired serological response using univariable analyses.
| Parameter | Impaired response rate | Odds ratio | Univariable analysis | p-value | Odds ratio | Multivariable analysis | p-value |
|---|---|---|---|---|---|---|---|
| 95% confidence interval | 95% confidence interval | ||||||
| Allo-HCT | |||||||
| Age (≥60y vs. <60y) | 35.2% (58/165) versus 21.5% (74/345) | 1.99 | 1.32–2.99 | 0.001 | 2.18 | 1.27–3.74 | 0.004 |
| Sex (male vs. female) | 29.3% (80/273) versus 21.9% (52/237) | 1.47 | 0.99–2.21 | 0.059 | 1.09 | 0.67–1.78 | 0.74 |
| Underlying diseases (lymphoid vs. others) | 28.7% (51/178) versus 24.4% (81/332) | 1.24 | 0.83–1.88 | 0.30 | |||
| Disease status (high-risk vs. standard-risk) | 32.2% (59/183) versus 22.6% (72/319) | 1.63 | 1.09–2.45 | 0.02 | 1.42 | 0.86–2.33 | 0.17 |
| Prior HCT (yes vs. no) | 32.5% (13/40) versus 25.3% (119/470) | 1.42 | 0.71–2.84 | 0.32 | |||
| Stem cell source (CB vs. others) | 25.1% (49/195) versus 26.4% (83/315) | 0.94 | 0.62–1.41 | 0.76 | |||
| Donor (HLA mismatched vs. matched) | 28.3% (95/336) versus 21.8% (37/170) | 1.42 | 0.92–2.19 | 0.12 | 1.96 | 1.10–3.45 | 0.02 |
| Conditioning (MAC vs. RIC) | 23.0% (76/330) versus 31.8% (55/173) | 0.64 | 0.43–0.97 | 0.03 | 0.77 | 0.45–1.32 | 0.35 |
| GVHD prophylaxis (CIs + sMTX vs. others) | 24.8% (69/278) versus 27.6% (62/225) | 0.87 | 0.58–1.29 | 0.49 | |||
| ATG use (yes vs. no) | 32.6% (15/46) versus 25.4% (116/457) | 1.42 | 0.74–2.73 | 0.29 | |||
| PTCy use (yes vs. no) | 39.3% (22/56) versus 24.4% (109/447) | 2.01 | 1.13–3.58 | 0.02 | 1.77 | 0.85–3.68 | 0.13 |
| Acute GVHD (II-IV vs. 0-I) | 28.6% (67/234) versus 23.8% (64/269) | 1.29 | 0.86–1.91 | 0.22 | |||
| Chronic GVHD (present vs. absent) | 41.5% (71/171) versus 18.0% (61/339) | 3.24 | 2.14–4.88 | <0.001 | 1.69 | 0.95–3.03 | 0.07 |
| Systemic steroid use (yes vs. no) | 50.7% (75/148) versus 15.8% (57/362) | 5.5 | 3.58–8.44 | <0.001 | 2.03 | 1.13–3.65 | 0.02 |
| Continued CI use (yes vs. no) | 46.5% (72/155) versus 16.9% (60/355) | 4.27 | 2.80–6.49 | <0.001 | 1.74 | 0.97–3.12 | 0.06 |
| Interval from HCT to vaccination (<12 m vs. ≥12 m) | 40.6% (26/64) versus 23.8% (106/446) | 2.19 | 1.27–3.78 | 0.005 | 1.57 | 0.77–3.21 | 0.22 |
| Absolute lymphocyte count (<1000/μL vs. ≥1000/μL) | 62.3% (33/53) versus 21.7% (99/457) | 5.97 | 3.28–10.85 | <0.001 | 2.87 | 1.37–6.01 | 0.005 |
| Number of CD4+ T-cells (<300/μL vs. ≥300/μL)a | 50.9% (29/57) versus 22.7% (103/453) | 3.52 | 2.00–6.19 | <0.001 | |||
| Number of CD8+ T-cells (<300/μL vs. ≥300/μL) | 25.0% (8/32) versus 26.0% (124/478) | 0.95 | 0.42–2.17 | 0.91 | |||
| Number of B-cells (<100/μL vs. ≥100/μL) | 45.1% (88/195) versus 14.0% (44/315) | 5.07 | 3.31–7.75 | <0.001 | 2.61 | 1.54–4.45 | <0.001 |
| Serum IgG level (<500/μL vs. ≥500/μL) | 74.2% (23/31) versus 22.8% (109/479) | 9.76 | 4.25–22.43 | <0.001 | 6.34 | 2.37–17.02 | <0.001 |
| Auto-HCTb | |||||||
| Age (≥60y vs. <60y) | 41.2% (7/17) versus 43.8% (7/16) | 0.9 | 0.23–3.58 | 0.88 | |||
| Sex (male vs. female) | 42.1% (8/19) versus 42.9% (6/14) | 0.97 | 0.24–3.92 | 0.97 | |||
| Underlying diseases (lymphoid vs. others) | 46.7% (14/30) versus 0.0% (0/3) | NC | |||||
| Disease status (high-risk vs. standard-risk) | 50.0% (2/4) versus 41.4% (12/29) | 1.42 | 0.17–11.51 | 0.74 | |||
| Prior HCT (yes vs. no) | NC (0/0) versus 42.4% (14/33) | NC | |||||
| Interval from HCT to vaccination (<12 m vs. ≥12 m) | 88.9% (8/9) versus 25.0% (6/24) | 24.0 | 2.48–233.5 | 0.006 | 15.5 | 1.29–865.5 | 0.03 |
| Absolute lymphocyte count (<1000/μL vs. ≥1000/μL) | 100.0% (2/2) versus 38.7% (12/31) | NC | |||||
| Number of CD4+ T-cells (<300/μL vs. ≥300/μL) | 87.5% (7/8) versus 28.0% (7/25) | 18.0 | 1.86–174.2 | 0.01 | 9.03 | 0.58–595.7 | 0.15 |
| Number of CD8+ T-cells (<300/μL vs. ≥300/μL) | 100.0% (1/1) versus 40.6% (13/32) | NC | |||||
| Number of B-cells (<100/μL vs. ≥100/μL) | 56.5% (13/23) versus 10.0% (1/10) | 11.7 | 1.27–108.2 | 0.03 | 4.47 | 0.27–288.04 | 0.47 |
| Serum IgG level (<500/μL vs. ≥500/μL) | 57.1% (4/7) versus 38.5% (10/26) | 2.13 | 0.39–11.59 | 0.38 | |||
- Abbreviations: ATG, anti-thymocyte globulin; CB, cord blood; CI, calcineurin inhibitor; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; MAC, myeloablative conditioning; PTCy, posttransplant cyclophosphamide; RIC, reduced-intensity conditioning; sMTX, short-term methotorexate.
- a Excluded from multivariable analysis due to collinearity with absolute lymphocyte count.
- b Exact logistic regression model was used for auto-HCT recipients due to the small number of impaired responders.
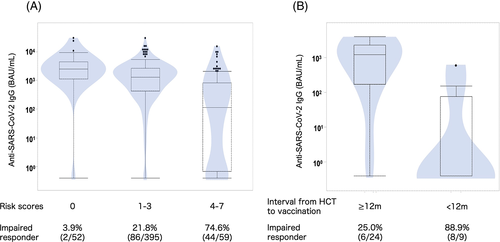
Subsequent multivariable analysis with excluding the number of CD4+ T-cells due to its strong collinearity to ALC demonstrated that the following parameters significantly correlated with impaired serological response: age (≥60 years vs. <60 years, odds ratio (OR) = 2.18 [95% confidence interval (CI), 1.27–3.74], p = 0.004), donor (HLA mismatched vs. matched, OR = 1.96 [95% CI, 1.10–3.45], p = 0.02), systemic steroid use (yes vs. no, OR = 2.03 [95% CI, 1.13–3.65], p = 0.02), ALC (<1000/μL vs. ≥1000/μL, OR = 2.87 [95% CI, 1.37–6.01], p = 0.005), number of B-cells (<100/μL vs. ≥100/μL, OR = 2.61 [95% CI, 1.54–4.45], p < 0.001), and serum IgG level (<500 mg/dL vs. ≥500 mg/dL, OR = 6.34 [95% CI, 2.37–17.02], p < 0.001) (Table 2). The correlations between these continuous variables and the titer of anti-SARS-CoV-2 IgG are shown in Figure S2. We constructed a risk scoring system by giving 2 points for serum IgG level (the highest OR) while 1 point for all others. Notably, this scoring system could stratify allo-HCT recipients into three groups; the rate of impaired serological response was 3.9% in the low-risk group (0 points, 10.3% in allo-HCT recipients), 21.8% in the intermediate-risk group (1–3 points, 77.5%), and 74.6% in the high-risk group (4–7 points, 11.6%) (Figure 1A).
3.6 Univariable and multivariable analyses for impaired serological response among Auto-HCT recipients
Among the 33 auto-HCT recipients, 14 (42.4%) patients were classified as impaired responders. According to the univariable analyses, factors related to decreased serological responses included interval from HCT to vaccination (<12 m vs. ≥12 m, OR = 24.0 [95% CI, 2.48–233.5], p = 0.006), number of CD4+ T-cells (<300/μL vs. ≥300/μL, OR = 18.0 [95% CI, 1.27–108.2], p = 0.01), and number of B-cells (<100/μL vs. ≥100/μL, OR = 11.7 [95% CI, 1.27–108.2], p = 0.03).
Multivariable analysis performed by exact logistic regression revealed that the interval from HCT to vaccination (OR = 15.5 [95% CI, 1.29–865.5], p = 0.03) was the sole statistically significant risk factor, although the number of CD4+ T-cells (OR = 9.03 [95% CI, 0.58–595.7], p = 0.15) showed a trend of relationship (Table 2). Impaired serological responses were observed in 8/9 (88.9%) patients who underwent vaccination within 12 months after HCT and 6/24 (25.0%) patients who were vaccinated more than 12 months after HCT (Figure 1B).
3.7 Incidence of COVID-19 during the study period
As mentioned above, fourteen patients (2.4%) were found to be seropositive at baseline and were excluded from the following analyses; 4 of these patients were symptomatic. Another case, who did not receive a second vaccine dose due to an AE, was diagnosed with COVID-19. Among the 543 patients who received two doses of vaccine, only 6 (1.1%) developed COVID-19 within 49 days of receiving the second dose. The anti-SARS-CoV-2 IgG titer in these patients varied from <0.8 to 9210 BAU/mL, and the incidence of COVID-19 was similar in the impaired responders (2/146, 1.4%) and responders (4/431, 1.0%).
3.8 Safety profiles
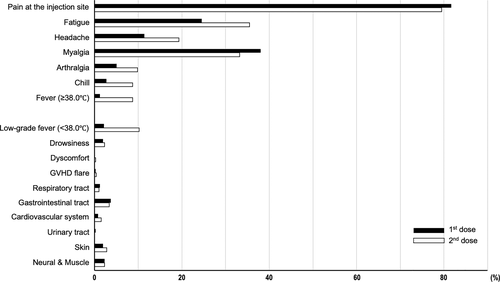
We could obtain information on AEs from 482 (88.8%) participants using specific questionnaires. As shown in Figure 2, the most frequent AE was pain at the injection site, which was observed in 81.7% and 79.5% of patients after the first and the second dose, respectively. The self-judged pain scales at the injection site were mild in most cases. Each systemic reaction (fatigue, headache, myalgia, arthralgia, chill, and fever) was found in 5%–40% of patients, with slightly increased incidence after the second dose. Other symptoms written by free format included low grade fever (<38.0°C, 2.1% after the first dose and 10.2% after the second dose), drowsiness (1.9% and 2.3%), GVHD flare (0.2% and 0.4%), respiratory tract symptoms (1.2% and 1.0%), gastrointestinal tract symptoms (3.7% and 3.3%), cardiovascular symptoms (0.8% and 1.5%), urinary tract symptoms (0.2% and 0%), skin symptoms (1.9% and 2.8%), and nerve and muscle symptoms (2.3% and 2.3%). These AEs were generally transient and tolerable.
4 DISCUSSION
In this large-scale multicenter prospective observational study, we demonstrated that SARS-CoV-2 mRNA vaccination provided adequate immunogenicity, without evident safety concerns for most HCT recipients. The observed seroconversion rate (90.4%) included a clinically relevant (≥250 BAU/mL) serological response rate of 73.1% and was consistent with previous reports showing 68%–96.5% seropositivity and 59%–87% clinically relevant titers.10-17, 21, 22 At the time point of serological test after the second dose, only 1.1% of patients in our cohort developed COVID-19. The incidence of COVID-19 was similar between responders and impaired responders, suggesting the contribution of cellular immunity to its prevention. A previous report evidenced vaccine-induced SARS-CoV-2-specific T-cell response among lymphoma patients treated with CAR-T therapy, leading to salvage the profound inhibition of humoral immune system.26
Among 552 patients receiving the first dose of vaccine, 5 (0.9%) patients did not receive a second dose due to vaccine-related events, including GVHD aggravation (n = 4) and AE (n = 1). Additional cases of GVHD flare occurred in 0.2% and 0.4% of patients after the first and the second dose, respectively. The rate of GVHD flare in our cohort was similar to that in previous reports varying 0%–11.5%.12, 27-30 These findings clearly indicate the safety of mRNA vaccines in HCT recipients with at least a 6-month interval from transplantation but not severe GVHD. The safety profiles of vaccinations for patients with shorter intervals and/or more severe GVHD should be investigated in the future studies.
We identified clinical and immunological parameters independently associated with impaired serological responses in allo-HCT and auto-HCT recipients. In allo-HCT recipients, multivariable analyses demonstrated that older age (OR:2.18, p = 0.004), HLA-mismatched donor (OR:1.96, p = 0.02), systemic steroids (OR:2.03, p = 0.02), lower number of ALC (OR:2.87, p = 0.005), lower number of B-cells (OR:2.61, p < 0.001), and lower serum IgG level (OR:6.34, p < 0.001) significantly correlated with impaired serological responses. Several previous reports also demonstrated a lower seroconversion rate for SARS-CoV-2 in elderly patients.31-33 Various mechanisms, including impaired memory B-cell formation, decreased class-switch recombination, defects in follicular dendritic cell activation/expansion, and more follicular regulatory T-cell: follicular helper T-cell (TFH cells), may contribute to attenuation of the germinal center response during aging, leading to a poor vaccine response.34
B-cells and its progeny, plasma cells, are the most important players in the generation of antibodies, and serum IgG levels correspond to the number and/or the function of B-cells. Therefore, the correlation between B-cell numbers and an impaired serological response is quite reasonable. Several previous studies have revealed that low B-cell numbers and/or low serum IgG levels correlated with less immunogenicity after vaccination.10, 13, 17, 21, 22, 30 Novel B-cell-targeted treatments, including CAR-T cell therapy and bispecific T-cell engager antibody, are also associated with low vaccine efficacy.17, 35
cGVHD may contribute to delayed/disrupted immune recovery by affecting many cell types, including B-cells, naïve CD4+ T-cells, TFH cells, and CD8+ T-cells. Immunosuppressants (e.g., systemic steroids and CIs) used for GVHD prophylaxis/treatment also inhibit the survival, proliferation, and activation of lymphocytes, resulting in lymphopenia with insufficient function. The current study demonstrated that continued use of systemic steroids (p = 0.02) or other immunosuppressants (p = 0.06) and cGVHD (p = 0.07) statistically or marginally correlated with impaired serological responses. These findings are consistent with previous studies in allo-HCT recipients,10, 12, 15-17, 22, 30, 32 solid organ transplant recipients,36 and patients with rheumatic diseases.37
HLA-mismatched donor was another risk factor for an impaired serological response. However, the proportions of impaired responders were similar in CB recipients (mostly from HLA-mismatched donors) and recipients of other stem cell sources (49/195, 25.1% vs. 83/315, 26.4% p = 0.76). CB grafts contain a higher proportion of naive T-cells but not antigen-specific memory T-cells and require a longer time for T-cell recovery. The slow T-cell recovery explains the higher susceptibility to viral infection, especially at early time points after transplantation. In contrast, rapid B-cell neogenesis has been reported compared with bone marrow transplantation.38 In addition, more chance of discontinuing CIs due to the lower incidence of cGVHD among CB transplant recipients may lead to a comparable serological response to other stem cell sources.
In auto-HCT recipients, a higher rate of impaired responses was observed compared with responses in allo-HCT recipients (42.4% vs. 25.9%). The interval from transplant to vaccination was the sole predictor of serological response (OR:15.5, p = 0.03) by using multivariable analysis. Patients with multiple myeloma were excluded from the current study; therefore, most patients (30/33) underwent auto-HCT for ML, with a history of rituximab treatment being 69.7%. Thus, the interval from transplant to vaccination may be parallel to that from the final dose of rituximab to vaccination, although detailed information was not available. B-cell reconstitution of the peripheral blood starts only 6 months after rituximab treatment,39 while a more profound recovery occurs later. Previous reports demonstrated that ML patients treated with anti-CD20 antibodies are unlikely to achieve serological response to SARS-CoV-2 vaccination and a longer time from last exposure predicted a higher positive rate with elevated antibody titers.40 In our cohort, all 3 patients who underwent auto-HCT for acute promyelocytic leukemia obtained clinically relevant antibody titers.
We also observed a trend between impaired serological response and the number of CD4+ T-cells in the multivariable analysis. CD4+ T-cells, including TFH cells, play an important role in antibody production via geminal center production and B-cell maturation. Canti, et al. showed that absolute counts of naïve CD4+ T-cells and TFH cells correlate with serological responses. Induction of antigen-specific TFH cell responses by mRNA vaccines was observed both in a mouse model41 and in patient samples.42 Antigen-specific TFH cell responses lead to the establishment of long-term immunity; thus, the lower number of TFH cells may account for the impaired serological responses in our study. However, these findings derived from small numbers of auto-HCT recipients should be interpreted carefully and might not be readily generalized.
There are several additional limitations to our study. The median interval from HCT to vaccination in the current cohort was 37 months; thus, obtained results were more pertinent to longer survivors with some degree of B-cell and/or T-cell recovery and less use of immunosuppressants. Lower seroconversion rate and more incidence of (severe) AEs or COVID-19 were estimated among patients with shorter interval from HCT or more frail condition. This analysis was focused on early postvaccination assessment. Thus, the longitudinal dynamics of antibody responses31 and long-term efficacy were not investigated. We did not assess neutralizing antibodies and cellular immunity against SARS-CoV-2, which also play a critical role in preventing COVID-19.43
In conclusion, SARS-CoV-2 mRNA vaccines exhibited good safety profiles under suitable conditions and robust serological responses in HCT recipients. Thus, transplant physicians should not hesitate to administer these vaccines to HCT recipients. However, the vaccine efficacy was insufficient for allo-HCT recipients with multiple risk factors (approximately ~15%) and auto-HCT recipients who underwent transplants within 12 months before vaccination. These risk factors correlate with a higher risk of mortality after the development of COVID-19 among HCT recipients,4 indicating that patients who require effective vaccination the most are less likely to launch a clinically relevant serological response. Alternative strategies, including a 3rd booster vaccination,10 prophylactic neutralizing antibody combination (tixagevimab-cilgavimab) therapy,44 and cellular therapies,45 should be considered for these high-risk patients during the continued SARS-CoV-2 pandemic.
AUTHOR CONTRIBUTIONS
Toshihiro Miyamoto, Koichi Akashi, and Koji Nagafuji designed and supervised this study. Yasuo Mori and Takuya Harada analyzed data, performed the statistical analyses, and wrote the first draft. All other authors participated in data collection, interpreted the analyzed data, and revised the manuscript. Yasuo Mori and Koji Nagafuji approved the final version of the manuscript for publication. The corresponding author attests that all listed coauthors meet authorship criteria.
ACKNOWLEDGMENTS
We are grateful to professor Koji Yonemoto (Ryukyu University) for the data analysis. We thank the medical and nursing staff who cared for the patients at the Fukuoka Blood and Marrow Transplantation Group and provided patients information. This study was partly supported by grant from Ishibashi Foundation for the Promotion of Science.
CONFLICT OF INTEREST
Junichi Sugita, Toshihiro Miyamoto, and Koichi Akashi received honoraria from Pfizer. The other authors have no conflicts of interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




