Allogeneic hematopoietic cell transplantation in older myelofibrosis patients: A study of the chronic malignancies working party of EBMT and the Spanish Myelofibrosis Registry
Funding information: This study was not economically funded.
Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is increasingly used in older myelofibrosis (MF) patients, but its risk/benefit ratio compared to non-transplant approaches has not been evaluated in this population. We analyzed the outcomes of allo-HCT in 556 MF patients aged ≥65 years from the EBMT registry, and determined the excess mortality over the matched general population of MF patients ≥65 years managed with allo-HCT (n = 556) or conventional drug treatment (n = 176). The non-transplant cohort included patients with intermediate-2 or high risk DIPSS from the Spanish Myelofibrosis Registry. After a median follow-up of 3.4 years, the estimated 5-year survival rate, non-relapse mortality (NRM), and relapse incidence after transplantation was 40%, 37%, and 25%, respectively. Busulfan-based conditioning was associated with decreased mortality (HR: 0.7, 95% CI: 0.5–0.9) whereas the recipient CMV+/donor CMV- combination (HR: 1.7, 95% CI: 1.2–2.4) and the JAK2 mutated genotype (HR: 1.9, 95% CI: 1.1–3.5) predicted higher mortality. Busulfan-based conditioning correlated with improved survival due to less NRM, despite its higher relapse rate when compared with melphalan-based regimens. Excess mortality was higher in transplanted patients than in the non-HCT cohort in the first year of follow-up (ratio: 1.93, 95% CI: 1.13–2.80), whereas the opposite occurred between the fourth and eighth follow-up years (ratio: 0.31, 95% CI: 0.18–0.53). Comparing the excess mortality of the two treatments, male patients seemed to benefit more than females from allo-HCT, mainly due to their worse prognosis with non-transplant approaches. These findings could potentially enhance counseling and treatment decision-making in elderly transplant-eligible MF patients.
1 INTRODUCTION
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm (MPN) affecting mainly elderly people, with a median age at diagnosis of over 65 years.1, 2 Median survival with conventional drug treatment is less than 5 years in patients with high-risk disease.1 Allogeneic hematopoietic cell transplantation (allo-HCT) constitutes the only curative treatment, but the advanced age of MF patients and the significant transplant-related mortality have historically limited the application of this procedure to less than 10% of MF patients.3, 4
There is an increasing use of allo-HCT in older patients with hematological malignancies, which has yielded reasonable results.5, 6 Regarding MF, two small retrospective series from academic centers7, 8 have shown favorable allo-HCT outcomes in elderly MF patients, particularly in those with minor comorbidities.8 These studies have demonstrated the feasibility of the procedure in selected older adults with MF, but a comparison of allo-HCT results with non-transplant approaches is lacking in this age group.
Our objective was to evaluate the outcome of allo-HCT in a large series of MF patients aged 65 years or older. In addition, we aimed to determine the excess mortality associated with allo-HCT vs non-transplant approaches in this age population. Such information may be useful to enhance understanding of the risk–benefit ratio of allo-HCT in older MF patients.
2 METHODS
2.1 Transplant cohort
EBMT is a scientific society representing more than 550 transplant centers. All patients whose transplant data are reported to EBMT provide informed consent for this information to be used in anonymized research projects. For the present study, inclusion criteria were MF patients undergoing first allo-HCT between January 2000 and December 2017 at an age ≥65 years. Patients with history of leukemic transformation were excluded as were those from countries reporting less than five cases. A total of 556 patients from 148 centers (12 countries) fulfilled the selection criteria, representing 14% of all transplanted MF patients reported to EBMT during the study period.
2.2 Non-transplant cohort
The Spanish Myelofibrosis Registry is a nationwide registry contributed by centers affiliated to the Grupo Español de enfermedades Mieloproliferativas Filadelfia Negativas (GEMFIN). Informed consent for inclusion in the registry is obtained in accordance with local research ethics committee requirements. Between January 2000 and December 2016, 1000 patients diagnosed with MF in 61 centers were included in the registry database. A total of 616 patients were aged 65 years or more at MF diagnosis, among which 299 were assigned to the intermediate-2 or high risk DIPSS9 categories. Three cases undergoing allo-HCT during the observation period were excluded. The final non-transplant study cohort comprised 176 patients with age within the same range than the EBMT cohort (65 to 76 years; median 72 years). Treatments given to these patients were: red blood cell transfusions (n = 96, 54.5%), hydroxyurea (n = 93, 53%), erythropoiesis-stimulating agents (n = 86, 49%), ruxolitinib (n = 44, 25%), danazol (n = 42, 24%), immunomodulating agents (n = 17, 10%; thalidomide: n = 9, lenalidomide: n = 8), corticosteroids (n = 16, 9%), splenectomy (n = 6, 3%), spleen irradiation (n = 3, 2%), and interferon (n = 1, 1%).
2.3 Study definitions and variables
For the present study, primary and secondary graft failure were considered together.10 Cases with relapse within 1 month of graft failure or with graft loss documented later than 180 days post-transplant were coded as relapse and not as graft failure.11 Patients were considered at risk for graft-versus-host disease (GVHD) if they engrafted. Acute GVHD was scored according to Glucksberg et al.12 and chronic GVHD according to the criteria of Shulman et al.13 Disease progression/relapse was defined as disease persistence or recurrence in patients who survived more than 28 days after transplantation.14 In patients who died after disease relapse, relapse was considered the primary cause of failure, regardless of the immediate cause of death.15
Variables investigated for prognostic significance after allo-HCT were the following: calendar year at transplantation (<2010 vs later), patient sex, age ≥68 years, Karnofsky Performance Status (KPS, <90% vs ≥90%), Hematopoietic Cell Transplantation-specific Comorbidity Index16 (HCT-CI, ≥3 vs other), MF subtype (primary vs secondary), DIPSS risk category (high risk vs others),9 driver mutation status, ruxolitinib treatment before transplant, intensity of conditioning regimen (MAC, myeloablative vs RIC, reduced intensity), type of conditioning (busulfan-based vs melphalan-based vs others), antihuman T-lymphocyte immunoglobulin (ATG) use, donor/patient CMV serostatus, donor/patient sex (female donor to male recipient vs any other), donor type (unrelated vs related), donor/recipient HLA match (matched vs mismatched), and source of progenitor cells (peripheral blood vs bone marrow).
2.4 Statistical methods
Primary study outcomes were death, relapse, non-relapse mortality (NRM), relative survival and excess mortality. Non-relapse mortality was defined as death without relapse/progression. In GVHD-free and relapse-free survival (GRFS) calculation, grade 3–4 acute GVHD, extensive chronic GVHD, relapse, and death were considered as relevant events.17 Survival curves were drawn by the Kaplan–Meier method. Median follow-up was determined using reverse Kaplan–Meier method. Cumulative incidence was used to estimate risk of relapse and GVHD in the framework of competing events. Death and relapse were taken as competing events for GVHD, whereas NRM competed with relapse risk. Prognostic factors for these outcomes were analyzed by estimating the sub-hazard ratio (SHR),18 which is the risk yardstick used in competing risks analysis and equivalent to hazard ratios in Cox regression.
Modeling excess mortality allows capturing not only deaths directly caused by the disease in question but also indirect deaths caused by the treatment or the interaction between the disease's phenotype and other comorbidities (eg, anemia and cardiac diseases). Excess mortality enables comparison of mortality rates across different countries and distant time periods after discounting changes in the population life-expectancy and the demographic effects of age and sex.19 Estimates of expected mortality in the general population were derived from life tables for participating countries stratified by age, sex, and calendar year, obtained from the Human Mortality Database [www.mortality.org]. Time at risk was defined as starting at MF diagnosis in patients managed with non-transplant treatment (GEMFIN cohort) and at date of allo-HCT in patients undergoing HCT (EBMT cohort). Onset of risk in EBMT and GEMFIN cohorts was based on the assumption that patients within the same DIPSS category have similar prognosis, regardless prior follow-up time.2
Excess mortality was expressed as incidence rates (IR) in number of events per 100 patient-years of follow-up that were statistically compared by calculating the excess mortality ratio as described by Dickman and Coviello.20 All calculations were performed with IBM SPSS, version 26 and Stata 11 (www.stata.com).
This retrospective study was approved by the Chronic Malignancies Working Party (CMWP) of EBMT.
3 RESULTS
3.1 Patient characteristics at time of transplant
The main characteristics of the 556 patients are shown in Table S1. Median age at transplantation was 67 years (range, 65–76) and 68% were male. Median time from MF diagnosis to transplant was 2.5 years (IQR: 0.8–8.2). Among the 241 patients with data available on DIPSS category at HCT, most (83%) belonged to the intermediate-2 or high risk groups. Conditioning regimens were mainly of reduced intensity (75%) and busulfan-based (67%). A total of 387 patients (70%) received a graft from an adult unrelated donor.
The proportion of allo-HCT performed in MF patients aged ≥65 years in the EBMT database increased markedly over time, accounting for 2%, 9%, and 19% of total transplants for this indication during the time periods 2000–2005, 2006–2011, and 2012–2017, respectively.
3.2 Survival, complications and causes of death after transplant
After a median follow-up of 3.4 years, 306 (55%) patients had died. The estimated median survival was 2.1 years (95% CI: 1.4–3.3), and estimated survival rates at 1, 3, and 5 years were 59%, 49%, and 40%, respectively (Figure 1(A)).
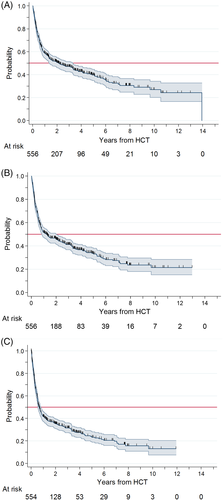
Cumulative incidence of graft failure was 6% at 6 months. Cumulative incidence was 23% for grade II-IV and 11% for grade III-IV acute GVHD at 180 days. Cumulative incidence of any grade and extensive chronic GVHD at 2 years was 33% and 18%, respectively.
Causes of death were GVHD (n = 106), relapse/progression (n = 80), infection (n = 69), organ failure/toxicity (n = 10), second malignancy (n = 6), and other (n = 15). Cause of death was unknown in 20 patients.
3.3 Prognostic factors for survival after transplant
The unadjusted association between patient and procedure characteristics and mortality risk after allo-HCT is illustrated in Figure S1. Predicted 5-year survival was better in patients receiving busulfan-based conditioning (n = 369) than in those receiving melphalan-based conditioning (n = 78) (42% vs 33%, respectively; HR: 0.6, 95% CI 0.5–0.9; p = 0.004) (Figure S2).
After multivariate stepwise selection, the only factors remaining in the model associated with higher mortality were JAK2-positive genotype (HR: 1.9, 95% CI: 1.1–3.5; p = 0.03) and donor-negative/patient-positive CMV serostatus (HR: 1.7, 95% CI: 1.2–2.4; p = 0.002), whereas receiving busulfan-based conditioning (HR: 0.7, 95% CI: 0.5–0.9; p = 0.04) predicted lower mortality compared to melphalan-based regimens.
3.4 Non-relapse mortality
The estimated risk of NRM at 1, 3, and 5 years was 27%, 33%, and 37%, respectively (Figure S3). Factors independently associated with increased NRM were the donor-negative/patient-positive CMV serostatus combination (SHR: 2.1, 95% CI: 1.4–3.2; p = 0.001) and the JAK2-positive genotype (SHR: 2.6, 95% CI: 1.0–2.5; p = 0.04). In contrast, busulfan-based conditioning (SHR: 0.5, 95% CI: 0.3–0.8; p = 0.001) predicted lower NRM compared to melphalan-based regimens (Table S2). Also, NRM was lower with busulfan when the comparison group was restricted to the 78 patients conditioned with melphalan-based regimens (SHR 0.5, 95% CI: 0.3–0.7; p < 0.001).
3.5 Relapse/progression after HCT
Cumulative incidence of relapse/progression at 1, 3, and 5 years was 18%, 22%, and 25%, respectively (Figure S3). In the univariable analysis, the only factor significantly associated with lower relapse incidence was the use of a matched unrelated donor (SHR: 0.50, 95% CI: 0.33–0.77; p = 0.002) (Table S2). When the analysis was restricted to patients conditioned with either busulfan- or melphalan-based regimens, those receiving busulfan had increased relapse risk (SHR 2.0, 95% CI 1.0–3.8; p = 0.04).
Figure 1(B) and (C) show the progression-free survival and the GRFS, respectively. The probability of surviving free of progression after 1, 3, and 5 years was 55%, 44%, and 36%, respectively. These figures decreased to 42%, 32%, and 23%, respectively, when we considered the probability of being free of severe acute or chronic GVHD.
3.6 Excess mortality associated with allo-HCT and with conventional drug treatment
We compared excess mortality between the 556 transplanted patients and a cohort of 176 patients from the Spanish Registry of Myelofibrosis, aged 65 to 76 years with intermediate-2/high risk DIPSS at MF diagnosis. After a median follow-up of 4.6 years (95% CI: 3.1–5.3) from diagnosis, the estimated 5-year survival rate of the non-transplant cohort was 33% (95% CI: 25–42) (Figure S4). As shown in Table S3, transplanted patients were on average 5 years younger and predominantly male compared to the non-HCT group.
Figure 2 shows the observed and expected mortality in both patient cohorts, as well as the estimated relative mortality up to 8 years from either date of allo-HCT in transplanted patients or date of MF diagnosis in the non-HCT group. It is worth noting that relative mortality curve of transplanted patients did not become horizontal within the first 8 years of follow-up, which reveals persistently increased mortality over the death rate observed in the matched general population.

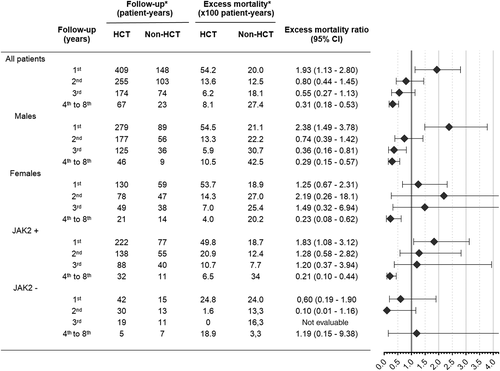
We next analyzed the impact on excess mortality of those prognostic factors identified in the HCT group that could be evaluated in the non-HCT cohort, namely sex and JAK2 genotype. Figure 3 illustrates the excess mortality associated with HCT as compared to non-HCT at several follow-up time points, categorized by sex and JAK2 genotype, as well as the excess mortality ratios after comparing both mortality rates. Three points should be underscored in Figure 3. First, allo-HCT shows higher early excess mortality independently of the prognostic group. Second, males did particularly well with HCT after the second follow-up year, as compared with non-HCT, whereas females did not benefited from HCT until after the fourth post-transplant year. Third, no definite effect could be ascribed to the JAK2 genotype on the excess mortality ratios.
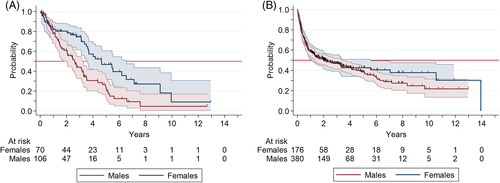
We next investigated the prognostic impact of sex in the non-HCT and the HCT cohorts. Male sex was associated with significantly increased mortality in non-HCT patients (HR: 2.01, 95% CI: 1.31–3.08; p = 0.001) after adjustment for age and DIPSS category (Table S4). Conversely, sex had no influence on prognosis in the HCT group, as previously shown in Figure S1. Survival curves by sex with the two treatment approaches are shown in Figure 4.
Time elapsed from MF diagnosis to HCT may have selected a group with particularly good prognosis not accounted for by the DIPSS, since patients had to survive until the procedure (length-time bias). Alternatively, it is conceivable that these patients had already spent some of the life expectancy determined by the disease, and might actually have a worse prognosis at the time of HCT. To investigate this possible source of bias, we compared survival in transplanted patients according to whether the time elapsed from diagnosis to HCT was <12 months (n = 160; median survival: 1.4 years, 95% CI: 0.8–4.1) or ≥ 12 months (n = 396; median survival: 2.3 years, 95% CI: 1.4–3.4) and found no significant difference (p = 0.38).
4 DISCUSSION
The present study evaluates allo-HCT outcomes in a large multicenter series of MF patients aged 65 years or older. Overall, 40% were alive at 5 years after transplantation, a lower figure than the 50% to 65% long-term survival reported in recent series of allo-HCT in all-age MF patients.10, 14, 21-24 Increased NRM (37% at 5 years), mostly attributed to GVHD, was the main cause of worse survival, as the relapse rate (25% at 5 years) was comparable to that reported in younger MF patients.10, 14, 22-26 Busulfan-based conditioning was associated with lower NRM and improved survival, whereas harboring the JAK2 mutation and the CMV-negative donor to CMV-positive recipient combination implied higher NRM and shorter overall survival. None of these factors had any influence on relapse risk. Of note, other well-recognized prognostic factors of allo-HCT outcomes in MF, such as performance status, comorbidity index or donor type, were not found to impact on survival.
A preparative regimen based on busulfan was the preferred option across the 148 participating centers. This conditioning regimen was associated with improved survival due to less NRM, despite a slightly higher relapse rate when compared with the second most common conditioning scheme based on melphalan. In previous retrospective studies27, 28 comparing busulfan-based and melphalan-based RIC regimens in all-age MF patients, the latter was associated with lower relapse incidence that was counterbalanced by higher NRM, resulting in similar survival. This therefore tips the balance in favor of preferential use of busulfan-containing regimens in elderly MF patients due to their greater vulnerability to treatment-related toxicity.
In our study, the recipient CMV+/donor CMV− combination was an independent predictor of increased NRM, in accordance with previous studies.29, 30 Receiving a graft from a CMV+ donor can likely reduce antivirals use in a CMV-negative recipient by increasing levels of multifunctional CMV-specific T cells,31 thereby reducing NRM. In addition, JAK2 mutated genotype was associated with increased NRM and shorter survival, with no significant effect on relapse risk. Discrepant results regarding the prognostic impact of driver mutations in transplanted MF patients have been reported,10, 32-36 with some authors describing better prognosis in CALR-10, 34, 35 or MPL-mutated36 patients. Consistent with our findings, the non-CALR/MPL genotype was considered a poor prognostic factor in the recently developed Myelofibrosis Transplant Scoring System (MTSS).29
Our data show that use of allo-HCT in older adults with MF is on the rise, in line with a trend observed in other hematological malignancies.5, 6 Nevertheless, it is worth noting that only highly selected elderly MF patients undergo allo-HCT in clinical practice. In the updated series of the Spanish Registry of Myelofibrosis, for instance, only nine patients (1.7%) eventually underwent allo-HCT among 535 MF patients ≥65 years assigned at diagnosis to the intermediate-2 or high risk IPSS37 groups, after a median follow-up of 30 months.
Before expanding the use of allo-HCT in the elderly MF population, it is critical to determine whether the consensus criteria for candidate selection38, 39 are appropriate in older patients, taking into account their shorter life expectancy and increased frailty owing to comorbidities.40 Accordingly, we aimed to compare the potential risk/benefit of allo-HCT and conventional drug treatment in MF patients aged 65 or older by estimating the excess mortality associated with each treatment modality. Excess mortality makes survival data from different countries and epochs better comparable and allows adjustment for demographic differences between HCT and non-HCT groups. Indeed, small differences in baseline life expectancy among countries or between men and women account for a large fraction of the observed survival in the elderly,41 which might have biased crude survival estimates against males, older patients, and those from countries with shorter life expectancies.
As expected, MF was associated with a large loss of life expectancy as compared to matched general population. Allo-HCT provided better long-term survival than conventional non-transplant approaches, though at the cost of increased early mortality, a “curve-crossing” phenomenon already reported in all-age MF patients.42, 43 It is worth noting that even 8 years after the procedure, transplanted patients die at a higher rate than their matched counterparts in the general population, as recently reported in all-age MF patients.44
We next ought to identify a particular patient profile more likely to benefit from allo-HCT. During the first 2 years, HCT was associated with higher excess mortality regardless of patient characteristics. In the long-term, JAK2-unmutated patients did better with both treatment modalities. On the other hand, males did particularly worse than women with conventional drug treatment after adjusting for age and DIPSS category. Several factors may explain why men benefited less from conventional treatment, a feature that has been observed in other MPN45, 46 and myelodysplastic syndromes.47 First, severe age-related comorbidities are more prevalent in men48 and can interact with the MF phenotype to increase long-term mortality (eg, cardiovascular disease and chronic anemia). In fact, chronic anemia is a known risk factor for increased morbidity and mortality in non-selected elderly populations,49 and it has also been associated with increased cardiac mortality in myelodysplastic syndromes.50 Second, male MPN patients are more prone to acquire additional high-risk mutations, independent of age, phenotype, and driver mutation, which has been linked to disease progression.46 Finally, the DIPSS9 model overrates the prognostic significance of anemia in females by using the same Hb threshold (<10 g/dl) in both sexes, despite the physiologically lower values in women.50, 51 This may be particularly relevant in older MF patients in whom anemia can be sufficient to classify the patient into the intermediate-2 risk category, a source of bias that new prognostic classifications are trying to fix.52
The present study has several limitations, of which the main one is that our transplantation results derive from a highly selected MF population and may not necessarily apply to elderly patients with other clinical characteristics. We also acknowledge that a controlled clinical trial would be the ideal setting to compare the results of allo-HCT and conventional drug treatment, but this type of study is unlikely to be feasible. Second, we assumed that disease severity at time of HCT was comparable to that of patients with the higher risk DIPSS categories at MF diagnosis. This assumption may however have biased the results in favor of HCT due to “selection by indication” (the best prognosis patients may have been selected for transplant), “length-time bias” (patients had to survive until the HCT) or because some patients were actually in the lower risk DIPSS categories at transplant. Third, it is possible that residual demographic effects not adjusted through estimation of excess mortality had favored the younger HCT cohort. Nevertheless, we could reasonably rule out length-time bias and previous studies have shown that DIPSS stratification is suboptimal to predict post-HCT survival.10, 33, 53
In conclusion, allo-HCT provides better long-term survival than conventional drug treatment in selected elderly patients with MF, albeit at the cost of increased short-term mortality. Busulfan-based RIC regimens can be considered the standard of care in this specific population. Male patients seem to benefit more from HCT than females, mainly due to their worse prognosis with non-HCT therapies. Strategies aiming to reduce transplant-related mortality, such as the use of letermovir54 to prevent clinically significant CMV infection or the implementation of less toxic conditioning regimens (eg, treosulfan-based55) or more effective therapies for GVHD prevention (eg, post-transplant cyclophosphamide24), might help to expand the benefit of allo-HCT in the elderly MF population.
ACKNOWLEDGMENTS
We are indebted to all centers participating in the EBMT and GEMFIN databases. The list of contributing centers is shown in the supplemental material.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




