Post-transplantation donor-derived Sezary syndrome in a patient with A91V PRF1 variant hemophagocytic lymphohistiocytosis
A 22-year male with a history of infectious mononucleosis (age 12), herpes zoster infection (age 14), C282Y homozygous hereditary hemochromatosis, and no significant family history was diagnosed with Epstein–Barr virus (EBV)-related hemophagocytic lymphohistiocytosis (HLH) when he presented with fever, pancytopenia, splenomegaly, hyperferritinemia (>40 000 ng/mL), hypertriglyceridemia, high soluble IL2 receptor alfa (sIL2R; 13 994 pg/mL) and hemophagocytosis on bone marrow (BM). Polymerase chain reaction (PCR) showed >1.5 million EBV copies/mL. He was treated with rituximab, dexamethasone, and etoposide with response, and after 8 weeks of therapy, he presented to our center for further management. At that time, his white blood count was 16 × 103/μL (range 4.0–11.0 × 103/μL), absolute neutrophil count 13 × 103/μL (1.70–7.30 × 103/μL), hemoglobin 9 gm/dL (12.0–16.0 gm/dL), platelets 476 × 103/μL (140–440 103/μL), serum creatinine 1.63 mg/dL (0.67–1.17 mg/dL), ALT 233 U/L (≤41 U/L), alkaline phosphatase 154 U/L (40–129 U/L), total bilirubin 1.7 mg/dL (<1.3 mg/dL), fibrinogen 321 mg/dL (202–450 mg/dL), ferritin 4219 ng/mL (30–400 ng/mL), LDH 637 U/L (30–400 ng/mL), triglycerides 371 mg/dL (≤149 mg/dL), sIL2R 852 pg/mL (≤1033 pg/mL) and PCR showed <400 EBV copies/mL. The PET-CT scan was unremarkable with hepatosplenomegaly no longer appreciated. Bone marrow biopsy showed 40% cellularity, trilineage hematopoiesis, no hemophagocytosis, and normal diploid karyotype. Flow cytometry showed virtually absent B-cells and no aberrant T-cells. Next-generation sequencing (NGS) using an 81-gene panel for myeloid malignancies did not identify any somatic mutations. Genetic testing for genes associated with familial HLH, EBV susceptibility, and immunodeficiency identified a heterozygous PRF1 gene variant (c.272C > T [p.Ala91Val]).
Around 3 months from original diagnosis, while on dexamethasone taper at 1.25 mg daily dose, his HLH relapsed with EBV reactivation (viral load 107 087 copies/mL), with recurrent fevers, pancytopenia, hyperferritinemia (71 611 ng/mL), elevated sIL2R (1711 pg/mL) and BM biopsy now with identificable hemophagocytosis. Flow cytometry showed normal lymphocytes. Extensive testing excluded other infectious, rheumatologic, and malignant etiologies. He was re-induced with rituximab, etoposide, dexamethasone, and tociluzumab on a clinical trial (NCT02385110). Post-treatment EBV was undetectable. Thereafter, he underwent A-antigen mismatched allogeneic hematopoietic stem cell transplantation (HCT) using an EBV-seropositive unrelated female donor (matched at -DRB3/4/5 and DPB1 loci). Bone marrow biopsy pre-HCT showed no hemophagocytosis, flow cytometry showed normal lymphocytes, and PCR showed no monoclonal T-cell receptor gamma (TCRg) or T-cell receptor beta (TCRb) gene rearrangements. He received fludarabine, melphalan, and alemtuzumab reduced-intensity conditioning (RIC), with tacrolimus and methotrexate for graft vs host disease (GVHD) prophylaxis. On day −1, he experienced another episode of EBV-related HLH flare (viral load 20 652 copies/mL and ferritin 89395 ng/mL) for which he received rituximab and intravenous immunoglobulin. His transplantation course was further complicated by Stenotrophomonas and Escherichia coli pneumonia treated with antimicrobials per sensitivities. He recovered, engrafted neutrophils on day +16, and was successfully discharged. Fluorescence in-situ hybridization at day 30 showed 100% female cells, and microsatelite polymorphism analysis (chimerism) revealed 100% donor myeloid, T-cells, and NK-cells.
All post-HCT EBV PCRs remained undetectable. He had mild upper gastrointestinal tract GVHD about 2 months post HCT treated with budesonide. A BM biopsy at day 60 was normal with no aberrant T cells or NK-cells on flow cytometry, normal stains for cytotoxic markers (granzyme B, perforin, and TIA-1), diploid female karypotype, and 100% donor myeloid, T cells, and NK cells.
Five months post-HCT, he developed pneumonia with widespread airspace and interstitial opacities, respiratory failure requiring intubation, and acute kidney injury requiring continuous renal replacement therapy (CRRT). Infectious work-up was unrevealing. At that time his absolute CD4 count was 89 cells/μL, the absolute CD19 B cell count was 0 cells/μL, and absolute NK cells were normal (184 cells/μL). He recovered with empiric broad-spectum treatment, was successfully extubated, his intrinsic renal function returned and CRRT was discontinued, and he was discharged.
Two weeks later, he developed periocular faintly erythematous eczematous papules and scattered superficial pustules on the chest and abdomen. Skin biopsy was negative for GVHD and showed perifollicular inflammatory infiltrate with perivascular and interstitial lymphocytic infiltrate with numerous eosinophils, consistent with a dermal hypersensitivity reaction. He was treated with topical hydrocortisone and antibacterial soap showering, with which his symptoms improved.
About a month later (day +202), he developed diffuse xerosis and widespread ichthyosis on lower extremities and superimposed erythroderma with virtually 100% body surface area (BSA) involvement, and absolute eosinophilia (7.56 × 103/μL; range 0.04–0.40 × 103/μL). Skin biopsy showed lichenoid and spongiotic dermatitis with eosinophils, suggesting hypersensitivity drug reaction. Medication review revealed allopurinol and dapsone to be temporally related - potentially causing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Given extreme immunocompromised state and recent pneumonia, systemic steroids were deferred. He was treated with steroid wet wrap therapy and intensive nursing care with daily gentle skin cleansing. With this, and with discontinuation of allopurinol and dapsone, his eosinophilia normalized and skin improved.
Shortly thereafter (day +229), while still on therapeutic tacrolimus, he was admitted again with generalized exfoliative erythroderma. (Supplementary Figure 1(A) and (B)) Repeat skin biopsy showed dense perivascular and lichenoid lymphoid infiltrate with exocytosis with numerous dyskeratotic and apoptotic keratinocytes. Some of the intraepidermal lymphocytes revealed hyperchromasia and irregular nuclei. Immunohistochemical studies showed the lymphocytes to be predominantly CD3+ T-cells, mostly CD4+ dermal lymphocytes, with the intraepidermal lymphocytes expressing CD8 (Figure 1). Epstein–Barr encoding region (EBER) in-situ hybridization was negative. Note, PCR showed monoclonal TCRg chains and oligoclonal TCRb pattern. These findings were suggestive of Sezary syndrome-like clonal T-cell lymphoid proliferation. Peripheral blood (PB) flow cytometry showed aberrant T cells, constituting 36.6% of lymphocytes, CD4 + CD26- cells were 79.2% of lymphocytes, with an absolute CD4 + CD26- count 5344/μL and absolute CD4 count 6220 cells/μL. A BM biopsy showed 30%–40% cellularity and hemophagocytosis, and PCR for the first time now showed monoclonal TCRg chains (same as in skin) and oligoclonal TCRb pattern. Again, NGS showed no mutations. Chimerism studies showed 100% donor myeloid, T cells and NK cells. The PET/CT scan showed bilateral axillary lymphadenopathy - 1.6 × 2.0 cm (SUV 1.8) and 2.8 × 2.3 cm (SUV 2.5), no hepatosplenomegaly, and diffuse uptake in the skin. Lymph node biopsy showed atypical T cells immunophenotypically similar to that detected previously. Chimerism analysis showed 100% donor myeloid, T cells, and NK cells.
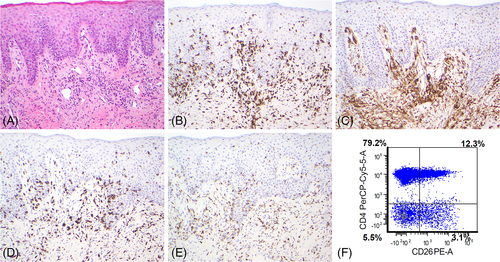
He met the diagnostic criteria for Sezary syndrome with generalized erythroderma, clonal T-cell population in the skin as well as BM, absolute Sézary cell count >1000 cells/μL (5344/μL), CD4 + CD26- ratio ≥ 30% (75.2%), and CD4:CD8 ratio > 10:1 (12:1).
Tacrolimus taper was started. In addition to intensive skincare (whirlpool therapy, topical mupirocin, silver sulfadiazine, steroid wet wraps, and moisturizers) and antibiotics for Staphylococcus aureus decolonization, he was treated with extracorporeal photopheresis (ECP) for about 2 months, which had to be discontinued due to recurrent line infections. He was then treated with bexarotene and total skin electron beam therapy 250 cGy twice weekly for 10Gy and then an additional 12Gy. He had transient skin improvement (Figure 1(C) and (D)). One month into treatment, his PB flow improved to 15% CD4+/CD26- (absolute count: 505 cells/μL), which normalized two months later with no aberrant T cells. This was confirmed on two more occasions 3 months apart. However, he continued to have skin involvement with cyclic diffuse exfoliative erythroderma, scaly patches with overlying coarse desquamation. (Supplementary Figure 1(C) and (D)) Repeat chimerism analysis confirmed 100% donor myeloid, T cells and NK cells. Given predominately skin involvement and clearing of PB, the plan was to start dupilumab (IL-4 receptor antagonist monoclonal antibody). However, with ongoing symptoms and recurrent infections, he opted for home hospice (day +473) and died (day +624). The donor remains healthy based on the information provided by the national donor registry with last follow-up about 2.5 years post HCT.
Detailed treatment of Sezary syndrome is discussed elsewhere,1 but generally, management includes skin-directed therapies (e.g., PUVA), ECP, biologic-response modifiers (e.g., bexarotene and interferon-alpha), histone deacetylase inhibitors (e.g., romidepsin, vorinostat), monoclonal antibodies and immunotoxins (alemtuzumab, brentuximab vedotin, mogamulizumab and systemic chemotherapy (CHOP-like regimens).
After allogeneic HCT, post-transplant lymphoproliferative disorders (PTLD) are rare with an incidence of about 1%. Most PTLD are of B-cell lineage and EBV-positive, while T-cell PTLD are exceedingly rare. On literature search, we identified five cases of post-HCT cutaneous T-cell PTLD,2-6 one of which presented as Sezary syndrome.2 All of these cases presented >3 years after HCT and had severe GVHD requiring immunosuppression, while it was diagnosed within 8 months in our case with no GVHD. Known predisposing risk factors for early-onset PTLD (<1 year) include mismatched/unrelated donor, T-cell deplete graft, GVHD prophylaxis using methods that deplete T-cell +/-NK cells (such as antithymocyte globulin or anti-CD3 monoclonal antibody) rather than methods that deplete both T and B cells (such as alemtuzumab). Our patient underwent mismatched unrelated donor HCT, but received T-cell replete graft and alemtuzumab.
Up to 40% of patients with familial HLH may have molecular alterations of the perforin (PRF1) gene, located on chromosome 10q22. The A91V variation has been considered a benign population polymorphism with <4% healthy individuals being heterozygous, although reports suggest it may be pathogenic.7, 8 Even heterozygous PRF1 defects can impair NK cell functional activity, but are presumed insufficient to abolish cytolytic activity completely and autosomal recessive inheritance of biallelic PRF1 pathogenic mutations are diagnostic of familial HLH.9, 10 It remains unanswered how our patient with rare diseases (homozygous C282Y, HLH, A91V perforin variation) developed an exceptionally rare post-HCT complication (Sezary syndrome). Whether this was causally related to A91V variant, which has been associated with NK/T-cell lymphomas,11 or related to skin flora providing chronic antigen stimulation leading to donor clonal T-cell expansion causing malignant transformation in a recipient with a genetic niche prone to immune dysregulation, and exaggerated antigen presentation by residual recipient dendritic cells, supplanted by intense immunosuppression (alemtuzumab and several courses of Rituximab), or purely a series of unfortunate events?
Open Research
DATA AVAILABILITY STATEMENT
Clinical de-identified data may be available on request from the corresponding author.




