Effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia patients and indirect comparison with rituximab-bendamustine: Results of study on 486 cases outside clinical trials
Valter Gattei and Massimo Gentile equally contributed as senior authors.
Ibrutinib (IB) has been initially approved for naïve patients' treatment with chronic lymphocytic leukemia (CLL) based on its superiority over chlorambucil.1 Two subsequent large phase 3 randomized trials demonstrated a longer progression-free survival (PFS) in IB treated cases compared to those receiving rituximab (R) combined with fludarabine (F) and cyclophosphamide (C, FCR)2 or with bendamustine (BR).3
Explorative analyses, demonstrating the superiority of IB over chemotherapy1 or chemoimmunotherapy2, 3 in cases with high-risk features, including unmutated IGHV (IGHV-UM) genes, del(17p) and/or TP53 mutations (TP53mut) and del(11q), justifies the attitude to limit FCR and BR only for low-risk and fit cases. However, these biomarkers' practical prognostic relevance in the era of new drugs remains an open issue.4 Results investigating the clinical impact of IB in the current clinical practice mainly focused so far on relapsed-resistant (RR) patients.5
Here, we conducted a multicenter, retrospective study to ascertain the predictive and prognostic relevance of well-known biological and clinical indicators in 165 patients treated with IB as first-line. In the same setting, we assessed the relative usefulness of IB versus BR, comparing the IB cohort with an additional retrospective multicenter cohort of 321 CLL cases treated with BR as first-line therapy outside clinical trials.
The baseline characteristics of the IB cases are listed in Table S1. The majority of patients were Binet stages B and C (89.7%). The median age was 71.8 years, 104 cases (63%) were males, and 70.2% of cases were IGHV-UM; moreover, del(17p) and TP53mut were observed in 43.6% and 38.8% of cases, respectively. Of note, patients with del(17p) were not included in the Resonate-2 trial1 or in FCR versus IB study,2 and only 5% to 6% of cases with del(17p) were accrued in the IB arms of the BR versus IB trial.3 Here, the incidence of del(17p) or TP53mut cases, due to IB prescription indications, supported a reliable explorative TP53 disruption sub-analysis. Forty-three patients (26.7%) discontinued IB, 24/43 for disease progression, including Richter's transformation (nine cases), and 19/43 for toxicity.
After a median follow-up of 31.6 months, 36 patients progressed or died, and 88.4% remained progression-free and alive at 1 year. On univariate Cox regression analysis, patients with anemia [Hazard ratio (HR) 2.0, 95% CI 1.0–3.8, p = .042], Binet C (HR 2.0, 95% CI 1.0–3.8, p = .043), del(17p) (HR 3.4, 95% CI 1.7–6.9, p = .001), and TP53 mutation (HR 2.4, 95% CI 1.1–5.1, p = .025) had a significantly higher risk of progression or death (Table S2). We performed two different multivariate Cox analyses in which either del(17p) (model 1) or a TP53 mutation (model two) were introduced together with anemia and Binet stage (Table S2). Notably, del(17p) (HR 3.1 95% CI 1.5–6.5, p = .002) in model one and TP53 mutations (HR 2.4 95% CI 1.1–5.2, p = .025) in model two, remained unique predictors independently associated with PFS (Table S2). Moreover, we tested the hypothesis of whether the concomitant presence of del(17p) and TP53 mutations, the latter representing 70.5% of del(17p) cases, could provide a more precise risk assessment. A Cox regression analysis adjusted for anemia and Binet stage showed a significantly inferior PFS (HR 4.5, 95% CI 1.7–11.7, p = .002) for cases with both TP53 mutation and del(17p) compared with those with a wild-type TP53 status, while the single TP53 gene alteration, either mutation or deletion, failed to significantly increase the risk of progression (HR 1.5, 95% CI 0.5–4.6, p = .4) (Figure S1).
Note, OS data revealed that 15/165 patients died, and 91.4% of cases were still alive at 2.5 years. Notably, none of the variables depicted in Table S2 were significantly associated with OS except that del(17p) (HR 4.1, 95% CI 1.3–13.3, p = .016). Again, cases with TP53 mutation and del(17p) disclosed a significant higher death risk (HR 5.5, 95% CI 1.7–25.8, p = .031) than the remaining groups of patients with unaltered or partially altered TP53 gene. Overall, our results suggest that the degree of TP53 function disruption [i.e., del(17p) or TP53mut versus del(17p) and TP53mut] appears to affect the response to IB in term of shorter PFS and possibly shorter OS. Our findings are germane to those of the largest cohort of IB-treated patients with del(17p),6 demonstrating an inferior PFS in cases with this chromosome abnormality.6 Notably, a landmark analysis evidenced that drug withdrawal predicted a significantly shorter OS than patients still on IB therapy, irrespective of whether discontinuation was driven by toxicity, disease progression, or Richter transformation (Figure S2). This finding is in keeping with previous reports indicating a poor outcome after IB discontinuation.7 The baseline characteristics of all patients who discontinued IB therapy for toxicity are listed in Table S3. A significantly higher rate of older patients was documented in this subset of patients (73.7% versus 54.1%, p = .02). New treatment regimens were initiated for 13/19 (68.4%) of the patients that discontinued IB, most commonly VEN-based (n = 9) or IDELA-based (n = 4) treatments.
Another aim of this study was to compare the IB cohort with an additional cohort of 321 cases treated with BR as first-line therapy outside clinical trials. In the BR cohort, we found a significantly higher proportion of cases with abnormal levels of both β2-microglobulin (β2-M) and lactic dehydrogenase (LDH), while IGHV-UM and del(17p) cases were more frequently observed in the IB cohort (Table S4).
An unadjusted Cox analysis performed in the combined cohort showed that IB was significantly more effective than BR in decreasing the risk of disease progression in treatment-naïve patients with CLL (Table S5). However, this analysis poses a hypothetical hitch of confounding by indication, that is, a bias that distorts the comparison between two treatments by the presence of an indication tilting the prescription toward a drug rather than another (herein, IB versus BR). To minimize the confounding by indication we adjusted the relationship between allocation therapy (IB versus BR) and disease progression for all the variables which resulted differently distributed between the two cohorts at study inception (see Table S4), as well as for all variables significantly associated with PFS at Cox univariate analysis, as described in Table S5. After jointly introducing these variables as covariates into a multiple Cox regression model, the protective effect of IB versus BR in terms of risk of disease progression (HR = 0.31, 95% CI 0.14–0.66, p = .002) was fully confirmed independently of a series of potential confounders (Table S6). Notably, del(17p) remained the only independent predictor of PFS (HR, 3.52; 95% CI, 1.83–6.78, p < .001) together with therapy allocation (Table S6).
We also investigated the interaction between the treatments under investigation, the presence/absence of del(17p), and PFS. In an unadjusted Cox regression analysis, patients treated with BR and harboring del(17p) had an HR of progression or death higher than that expected in the absence of interaction under the additive model, with a synergy index of 2.6 (Figure S3A). It means that the risk due to BR and del(17p) interaction was 2.6 times higher than that expected as a simple sum of the two risk factors' effects (i.e., by considering no interaction). These results did not change when the same analysis was carried out by adjusting for a series of potential confounders (Figure S3B).
To visually compare the IB versus BR PFS benefit consistently observed also for patients bearing del(17p), we constructed an additional multiple Cox model in which the variable representing the combination of the type of therapy and the del(17p) status was introduced together with Binet stage, β2-M, anemia, LDH, and IGHV mutational status. This analysis showed a clear overlap of PFS curves of del(17p) cases treated with IB with no-del(17p) cases treated with BR, while patients bearing del(17p) treated with BR experienced the worst outcome (Figure 1).
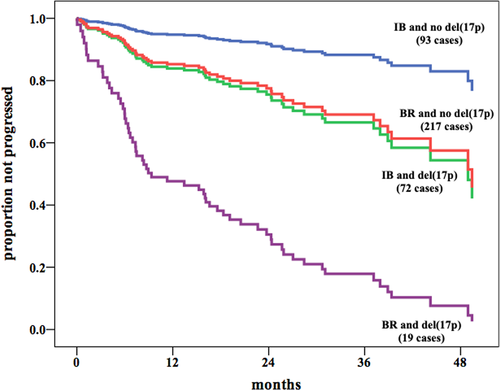
Finally, both the unadjusted and adjusted analysis of our cohort showed no significant differences between IB and BR in OS (data not shown).
Altogether, our retrospective multicenter analysis involving patients treated outside clinical trials, confirmed the superiority of IB over BR in terms of PFS, but not when OS was considered. Similarly, the ALLIANCE trial, while demonstrating a superior PFS in IB treated cases compared to cases treated with BR, fails to validate such superiority in the OS setting.3 Accordingly, less intensive chemotherapy with a combination of anti-CD20 and bendamustine given as front-line therapy has remained an additional choice for low-risk IGHV mutated (IGHV-MUT) fit cases without TP53 disruption.3 This short-term therapy could be envisaged during oncological counseling for a shared optimal clinical management of CLL low-risk patients in countries where this choice is allowed.
In conclusion, the results of this current clinical practice study demonstrated that IB therapy provides a superior PFS compared to BR, particularly in patients with del(17p). However, TP53 disruption still maintains its prognostic power in treatment-naïve patients with CLL treated with IB.
ACKNOWLEDGMENT
Associazione Italiana Ricerca sul Cancro (AIRC) Grant 5 × mille n.9980, (to F.M.); AIRC and Fondazione CaRiCal co-financed Multi-Unit Regional Grant 2014 n.16695 (to F.M.) Associazione Italiana Ricerca Cancro (AIRC), Investigator Grant IG-21687 (to V.G.) IG-5506 (to G.F.); Progetto Ricerca Finalizzata PE 2016–02362756 and RF-2018-12 365 790 (to A.Z.), Ministero della Salute, Rome, Italy (to V.G.); Compagnia S. Paolo, Turin, Italy (Project 2017.0526 to G.F.) and by the Ministry of Health (Project 5 × 1000, 2015 and 2016 and Current Research 2016 to G.F.). AIRC 5 × 1000 No. 21198 (to G.G.), Associazione Italiana per la Ricerca sul Cancro Foundation Milan, Italy and RF-2018-12 365 790, MoH, Rome, Italy (to G.G.). Funding of the project was provided by an unrestricted contribution from GILEAD Sciences Srl. The funding sources had no role in identifying statements, abstracting data, synthesizing results, grading evidence or preparing the manuscript, or in the decision to submit the manuscript for publication (ISR-17-10 250).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
F.M., M.G., M.F., G.D.P., F.R.M., D.R., F.D.R., G.G., L.T., A.P., R.F., and V.G. designed the study, analyzed and interpreted data, and wrote the manuscript; M.G., G.T., G.D., and F.M. performed statistical analysis; S.B., G.C., G.F., P.M., P.Me., F.M.R., A.Z., I.D.G., R.B., A.N., and M.F. performed central laboratory tests; G.R., P.S., L.L., M.C., Y.H., M.V., R.M., A.Ch., A.Co., R.Mo., A.V., D.P., G.L., U.C., I.S., E.V., E.A.M., R.C., A.R., I.A., A.B., S.G., H.A., and J.O. provided the patients and collected clinical data; and all authors gave final approval for the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request




