Intravenous high-dose methotrexate based systemic therapy in the treatment of isolated primary vitreoretinal lymphoma: An LOC network study
Funding information: The authors received no specific funding for this work.
Abstract
The treatment of primary vitreoretinal lymphoma (PVRL) remains controversial regarding the use of local, systemic, or combined treatments. The aim of this study was to analyze the efficacy and toxicity of intravenous high-dose methotrexate (IV HD-MTX) based systemic therapy in a uniformly treated population of PVRL patients. From a nationwide French database, we retrospectively selected 59 patients (median age: 70 years, median Karnofsky Performance Status: 90%) with isolated PVRL at diagnosis who received first-line treatment with HD-MTX between 2011 and 2018. 8/59 patients also received a local treatment. No deaths or premature discontinuations of MTX due to toxicity were reported. A complete response was obtained in 40/57 patients after chemotherapy. Before treatment, IL-10 was elevated in the aqueous humor (AH) or in the vitreous in 89% of patients. After treatment, AH IL-10 was undetectable in 87% of patients with a CR/uCR/PR and detectable in 92% of patients with PD/SD. After a median follow-up of 61 months, 42/59 (71%) patients had relapsed, including 29 isolated ocular relapses as the first relapse and a total of 22 brain relapses. The median overall survival, progression-free survival, ocular-free survival and brain-free survival were 75, 18, 29 and 73 months, respectively. IV HD-MTX based systemic therapy as a first-line treatment for isolated PVRL is feasible, with acceptable toxicity, even in an elderly population. This strategy seems efficient to prevent brain relapse with prolonged overall survival. However, the ocular relapse rate remains high. New approaches are needed to improve local control of this disease, and ocular assessment could be completed by monitoring AH IL-10.
1 INTRODUCTION
Primary vitreoretinal lymphoma (PVRL) is a rare malignancy that belongs to the larger group of primary central nervous system lymphomas (PCNSLs). PVRL affects mostly adults from the third to the eighth decades.1, 2 PVRL arises in the vitreous and/or retina and is most often a diffuse large B-cell lymphoma (DLBCL). Although often clinically indolent, the prognosis of the disease is poor and mainly related to the risk of relapse in the brain. Approximately 42% to 92% of PVRLs will have cerebral involvement within 8 to 37 months of diagnosis.3-5 Several studies have shown that the median overall survival of patients with isolated PVRL is much longer than that of patients who have intraocular disease associated with cerebral involvement (37-58 months compared to 18-34 months).3, 6-8
Although much progress has been made in the treatment of cerebral lymphoma, the treatment of isolated PVRL is still controversial because of the absence of prospective comparative studies regarding local treatment (i.e., intravitreal chemotherapy or ocular radiotherapy), systemic chemotherapy or a combination of local and systemic treatment. In 2011, the International Primary Central Nervous System Lymphoma Collaborative Group (IPCG) recommended local treatment for unilateral disease and local or extensive treatment (chemotherapy and local treatment) for bilateral disease.9 The British Neuro-Oncology Society recommended intravenous high-dose methotrexate (IV HD-MTX) combined with ocular and brain radiotherapy for the treatment of PVRL10 based on several studies of combined treatment in patients with PCNSL11, 12 and suggested intravitreal injection of methotrexate for patients with recurrent disease confined to the eyes as an effective option.13 In 2015, the European Association for Neuro-Oncology stated that either local or systemic treatments could be options for PVRL treatment and that the decision should be made according to the individual risk of treatment toxicities and local expertise. Ocular radiotherapy and intravitreal chemotherapy are associated with good response rates and a variable rate of ocular relapse (0%-61%) but do not seem to change the risk of secondary cerebral involvement and therefore overall survival.3, 14-19 Systemic chemotherapy seems promising in the prevention of cerebral involvement, as reported in several recent studies,8, 15, 20, 21 but therapeutic options included in the systemic chemotherapy arm were very different, and data about IV HD-MTX-based chemotherapy are scarce.
In France, a national expert network (the LOC network) was created in 2011 to standardize the treatment plan for PCNSL nationwide. This network issued national recommendations based on the literature regarding the management of PCNSL. For isolated PVRL, the recommendation was to treat all patients with fair general condition with IV HD-MTX based systemic therapy and to treat patients with worse condition with ocular radiotherapy or intravitreal MTX. Regarding follow-up, the recommendation was to perform an ophthalmological examination every 2 or 3 months during the treatment, then every 6 months during the first 2 years after the treatment, and once a year thereafter and to measure interleukin-10 (IL-10) levels in the aqueous humor (AH) at baseline, at the end of the treatment and in the case of any doubt of ocular relapse.
The aim of this study was to analyze ocular and systemic efficacy and toxicity in a population of patients with isolated PVRL who uniformly received first-line treatment with IV HD-MTX based systemic therapy.
2 METHODS
This work is based on an analysis of the French LOC network database,22 a nationwide database centralizing information from 28 expert centers for the management of PCNSL in France. The database was approved by the Institutional Ethical Committee of the coordinating center and by the French “Commission Nationale de l'Informatique et des Libertés” (CNIL). All patients gave informed consent for the submission of their data to the database. This study was conducted in accordance with the Declaration of Helsinki. No conflict of interest has been reported, and no private funds were used.
Patients were retrospectively selected according to the following criteria: (1) isolated PVRL at initial diagnosis (at baseline, all patients had at least a cerebral MRI and a full-body CT scan or FDG-PET scan to exclude cerebral and systemic involvement), (2) diagnosis confirmed by cytopathological examination of a vitreous sample, (3) age > 18 years, (4) immunocompetent status, and (5) IV HD-MTX-based chemotherapy as first-line treatment (MTX ≥1 g/m2). Patients with asymptomatic meningeal involvement were not excluded. Patients were selected in March 2018, and data were analyzed in September 2020.
The toxicity of chemotherapy was assessed according to the Common Terminology Criteria for Adverse Events version 5. Response to therapy was assessed according to the IPCG criteria23 with the following criteria: complete response (CR): no evidence of residual disease in the anterior chamber, vitreous or retina; uncertain complete response (uCR): minor nonspecific anomalies in ophthalmological findings; partial response (PR): >50% reduction in ophthalmological findings; progressive disease (PD): a worsening of the ocular findings or new ocular lesions; and stable disease (SD): none of the previous items. Ocular relapse was defined clinically by recurrent or new ocular disease in the anterior chamber, vitreous or retina. Assessment of ocular response can be difficult after vitrectomy and could only be classified as complete response or progression in some cases. IL-10 at diagnosis was considered elevated with a cutoff of 30 pg/ml in the aqueous humor and of 65 pg/ml in the vitreous for the diagnosis of PVRL according to previous publications.24-26 IL-10 in the AH was also monitored at the end of the treatment and during the follow-up at the discretion of the physicians. IL-10 levels were classified as detectable (≥2.5 pg/ml) or undetectable (<2.5 pg/ml) for the follow-up. Long-term responders were defined as patients who have never relapsed during the follow-up, with a minimum of 2 years-follow-up after first-line chemotherapy and non-responders were defined as patients who relapsed either during or at the end of first-line chemotherapy or within the 6 months after chemotherapy.
The four main endpoints were overall survival (OS), progression-free survival (PFS), ocular-free survival (OFS) and brain-free survival (BFS), calculated from the date of the cytopathological diagnosis. PFS was defined as the time without relapse (regardless of location) or without death (regardless of cause). BFS and OFS were defined as the time without brain and ocular relapse, respectively. Brain imaging was routinely performed once a year according to the national recommendation of the LOC network, and at any time in case of symptoms. Survival rates were calculated using the Kaplan–Meier method. The log-rank test was used to test for the equality of the PFS, BFS and OS distributions. A multivariate analysis was performed with the multivariate Cox proportional hazards regression model. Age and Karnofsky Performance Status (KPS) score, known as the main prognostic factors in PCNSL, were included in the multivariate analysis, as well as the variables with significant prognostic value in the univariate analysis. Two-sided p values < .05 were considered significant. All statistics were performed with xlstat software 2019.3.2.
3 RESULTS
3.1 Patients characteristics at diagnosis
Of the 1534 patients with PCNSL diagnosed between January 2011 and March 2018 included in the database, 69 patients had isolated PVRL (4.5%). Ten patients were excluded because they received only local treatment (N = 5) or non-IV HD-MTX-based systemic chemotherapy (N = 5). Fifty-nine patients (76% women) met the inclusion criteria. Their main characteristics are reported in Table 1. The median age was 70 years (range: 39-88), and the median KPS score was 90% (range: 60-100). There was bilateral ocular involvement in 39/59 (66%) patients. All the patients underwent vitrectomy. Eight of 59 patients had a second vitrectomy in the fellow eye because of a first negative vitrectomy (vitrectomy in 67 eyes). There was a pathological diagnosis of DLBCL in 97% of patients. In total, 89% (39/44) and 89% (42/47) of the patients had elevated IL-10 in the aqueous humor (AH) and vitreous, respectively. Lymphomatous cells were found in the CSF of 4/48 patients (8%).
| N | 59 |
|---|---|
| Age, median (range), years | 70 (39-88) |
| Sex M/F | 14/45 |
| Bilaterality | 39/59 (66%) |
| Initial visual symptoms | |
| Decreased visual acuity | 49/57 (86%) |
| Myodesopsia | 10/57 (18%) |
| Asymptomatic | 1/57 (2%) |
| Median best corrected visual acuity of affected eyes at diagnosis (95 eyes), logMAR | 0.22 (0-2.3) |
| Missing data | 2 patients (3 eyes) |
| Vitreous haze at diagnosis | 54/56 (96%) |
| Median grade of vitreous haze | 2 |
| Karnofsky performance status score, median (range) | 90% (60-100) |
| Median time to diagnosis (range), months | 8.7 (0.8-57) |
| Cytopathology | |
| Diffuse large B-cell lymphoma | 57/59 (97%) |
| Unclassifiable B-cell lymphoma | 2/59 (3%) |
| Lumbar puncture performed | 48/55 (87%) |
| Results of the lumbar puncture | |
| Normal | 38/48 (79%) |
| Lymphomatous cells (cytology or flow cytometry) | 4/48 (8%) |
| Uncertain results | 2/48 (4%) |
| Unknown results | 4/48 (8%) |
| IL6 and IL10 cytokines in the aqueous humor | |
| Number of data available | 44/59 |
| IL10 level (pg/ml): median (range) | 202 (2.5-8867) |
| Elevated IL10 (>30 pg/ml) | 39/44 (89%) |
| % of positive ISOLD score (Costopoulos et al. [35]) | 38/44 (86%) |
| IL10/IL6 ratio (>1) | 35/44 (80%) |
| IL6 and IL10 cytokines in the vitreous | |
| Number of data available | 47/59 |
| IL10 level (pg/ml): median (range) | 577 (2.5-6179) |
| Elevated IL10 (>65 pg/ml) (%) | 42/47 (89%) |
| % of positive ISOLD score (Costopoulos et al. [35]) | 36/39 (92%) |
| IL10/IL6 ratio (>1) | 37/39 (95%) |
| Type of treatment | |
| MTX ≥3 g/m2 | 52/59 (88%) (range 3-8) |
| MTX 1-2 g/m2 (mean 1.5 g/m2) | 7/59 (12%) |
| Median number of MTX injections | 6 (range 1-16) |
| Protocols | |
| (Rituximab), methotrexate, etoposide, carmustin, prednisone, (cytarabine) ((R)-MBVP-(A)) | 11/59 (19%) |
| (Rituximab), methotrexate, vincristine, procarbazine, cytarabine ((R)-MPVA) | 35/59 (59%) |
| (Rituximab), methotrexate, cytarabine | 11/59 (19%) |
| Other | 2/59 (3%) |
| Use of rituximab | 39/59 (66%) |
| Autologous stem cell graft | 1/59 (2%) |
| Associated local treatment | |
| Ocular radiotherapy 30 Gy | 6/59 (10%) |
| Intravitreal MTX | 2/59 (3%) |
- Abbreviations: IL-6, interleukin-6; IL-10, interleukin-10; MTX, methotrexate.
3.2 Treatment
The median duration between the diagnosis and the beginning of treatment was 48 days (range 3-229). The chemotherapy protocols are listed in Table 1. A total of 52/59 patients (88%) received ≥3 g/m2 IV HD-MTX per injection, with a median dose of 3 g/m2 (range 1-8 g/m2) and a median number of six injections. Rituximab (375 mg/m2) was administered to 39/59 patients (66%). A total of 8/59 (13%) patients received local treatment combined with systemic chemotherapy, consisting of consolidation ocular radiotherapy (RT) in six patients (30 Gray) and intravitreal MTX in two patients who received three injections and 37 injections, respectively.
3.3 Outcomes
The main outcomes are indicated in Table 2.
| Response to chemotherapy at 2 months, n (%) | |
| Complete or uncertain complete response (CR/uCR) | 21/52 (40%) patients |
| Partial response (PR) | 26/52 (50%) patients |
| Stable disease (SD) | 3/52 (6%) patients |
| Progressive disease (PD) | 2/52 (4%) patients |
| Assessable final response to first line chemotherapy, n (%) | 57 patients - 95 eyes |
| CR/uCR | 40/57 (70%) - 71/95 (75%) |
| PR | 3/57 (5%) - 4/95 (4%) |
| SD | 1/57 (2%) - 2/95 (2%) |
| PD | 13/57 (23%) - 18/95 (19%) |
| Missing data | Two patients (one death, one missing data) - three eyes |
| Visual acuity of affected eyes after chemotherapy, median, logMar (range) | 0.10 (0-2.3) |
| Improvement, n eyes (%) | 41/83 (50%) |
| Stability, n eyes (%) | 25/83 (30%) |
| Worsening, n eyes (%) | 17/83 (20%) |
| Missing data, n eyes | 15 |
| IL-10 in the aqueous humor at final response to first line chemotherapy (n = 37), n (%) | |
| In patients with CR/uCR | |
| Detectable IL-10 | 3/23 (13%) |
| Undetectable IL-10 | 20/23 (87%) |
| In patients with PR | |
| Detectable IL-10 | 0/1 (0%) |
| Undetectable IL-10 | 1/1 (100%) |
| In patients with SD/PD | |
| Detectable IL-10 | 12/13 (92%) |
| Undetectable IL-10 | 1/13 (8%) |
| Grade III-IV toxicities of chemotherapy, n (%) | |
| Myelosuppression | 27/51 (53%) |
| Lymphopenia | 14/51 (27%) |
| Neutropenia/neutropenia with fever | 19/51 (37%) / 3/51 (6%) |
| Thrombopenia | 14/51 (27%) |
| Anemia | 4/51 (8%) |
| Infections | 8/51 (16%) |
| Hepatic cytolysis | 4/51 (8%) |
| Renal dysfunction | 4/51 (8%) |
| Toxic deaths | 0/59 |
| Disruption of MTX due to toxicity | 0/59 |
| Ocular toxicity of local treatment | |
| Intravitreal MTX (n = 2) | |
| Cataract | 2/2 (100%) |
| Keratopathy | 1/2 (50%) |
| Ocular radiotherapy (n = 6) | |
| Dry eyes | 2/6 (33%) |
| Cataract | 3/6 (50%) |
| Radiation retinopathy | 1/6 (17%) |
| Macular edema | 1/6 (17%) |
| Number of patients who relapsed, n (%) | 42/59 (71%) |
| Only ocular relapse(s) | 17/59 (29%) |
| Only cerebral relapse(s) | 7/59 (12%) |
| Only systemic relapse(s) | 1/59 (1%) |
| Both ocular and cerebral relapse(s) | 15/59 (24%) |
| Ocular relapse first | 12/15 |
| Both ocular and cerebral relapse first | 2/15 |
| Cerebral relapse first | 1/15 |
| Ocular and systemic with/without brain relapse(s) | 2/59 (3%) |
| Side of ocular relapse | |
| Bilateral involvement at diagnosis | 24 |
| Bilateral relapse | 12/24 (50%) |
| Unilateral relapse | 12/24 (50%) |
| Unilateral involvement at diagnosis | 10 |
| Bilateral relapse | 3/10 (30%) |
| Unilateral relapse | 6/10 (60%) |
| In the same eye | 2 |
| In the other eye | 4 |
| Missing data | 1/10 (10%) |
| IL-10 at first ocular relapse (n = 27), n (%) | |
| Increase of IL-10 or detectable IL-10 | 25/27 (93%) |
| Undetectable IL-10 | 2/27 (7%) |
| Death, n (%) | 20/59 (34%) |
| Cause of death | |
| Lymphoma brain relapse | 17 |
| Cardiovascular disease | 1 (myocardial infarction during the first line chemotherapy) |
| Unknown | 2 |
3.3.1 Response to chemotherapy
At the end of chemotherapy, 40/57 patients (70%) and 71/95 (75%) eyes had a complete response (CR) or an unconfirmed complete response (uCR), while 13/57 (23%) patients and 18/95 eyes (19%) showed cancer progression. The response was not assessable in two patients (three eyes), due to a lack of data for one patient, and death due to myocardial infarction during the treatment for one patient. Best-corrected visual acuity improved in 41/83 affected eyes, worsened in 17/83 eyes, and remained stable in 23/83 eyes. Median visual acuity significantly improved after chemotherapy (logMar 0.10 after treatment versus 0.22 before treatment, p = .04). At the end of first-line chemotherapy, AH IL-10 levels were detectable in 3/23 patients (13%) with a CR or uCR (23 data points available among 40 patients with a CR/uCR); two of them relapsed in the eye 2 months after chemotherapy, and the 3rd patient experienced brain relapse 1 year after chemotherapy. The AH IL-10 levels were detectable in 12/13 patients with PD (median 31 pg/ml, range 5-594) and undetectable in 1/13 patients with PD whose IL-10 levels were never elevated. AH IL-10 levels were undetectable in 1/1 of PR patient who never relapsed.
3.3.2 Toxicity
Grade III-IV myelotoxicity was observed in 27/51 patients (53%). Only 3/51 (6%) patients had febrile neutropenia. Grade III-IV hepatic cytolysis and renal toxicity were observed in 4/51 (8%) and 4/51 (8%) patients, respectively. No ocular toxicity was reported from systemic chemotherapy. No deaths or disruptions of therapy due to toxicity were reported after the initial HD-MTX-based chemotherapy, but a reduction in the dose of MTX was reported in 5/51 (10%) patients due to grade III-IV renal or liver toxicities. The dose of cytarabine and vincristine were decreased in 3/52 (6%) patients and 2/52 (4%) patients, respectively. Otherwise, the patients completed all the treatments as per those treatment protocols. One case of endophthalmitis and one case of retinal detachment were reported following vitrectomy. No ocular complications due to anterior chamber puncture were reported. After intravitreal MTX, cataract and keratopathy were reported in 2/2 patients and 1/2 patients, respectively. After ocular radiotherapy, dry eyes, cataracts, radiation retinopathy and macular edema were reported in 2/6, 3/6, 1/6 and 1/6 patients respectively.
3.3.3 Relapses
With a median follow-up of 61 months (CI 95% 50-71), 42/59 (71%) patients relapsed during the follow-up. Thirty-four of 59 (58%) patients had at least one ocular relapse, including 17/59 (29%) patients who had only isolated ocular relapses and 22/59 (37%) patients who had brain relapse. For patients who had both ocular and cerebral relapses, the first relapse was ocular in 79% of the cases. Only one patient who initially had meningeal involvement experienced brain relapse 73 months after diagnosis. The only patient who has received high-dose chemotherapy with autologous stem cell transplantation (HCT-ASCT) in first-line treatment never relapsed and is still in complete remission 9.5 years after diagnosis.
Among patients whose first relapse was ocular (n = 33), AH IL-10 levels were available in 27 patients, and AH IL-10 levels were increased in 25/27 patients (93%) and were undetectable in 2/27 patients (7%). Of the six patients with ocular radiotherapy, none had ocular relapse, but three had brain relapse. The two patients who received intravitreal MTX never relapsed.
3.3.4 Survival
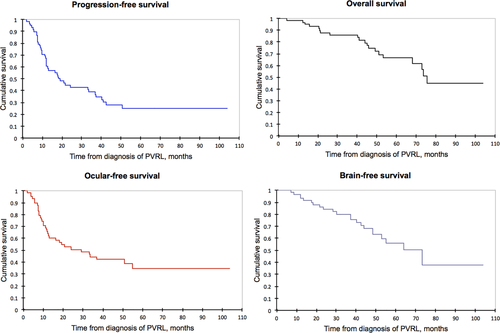
The median PFS was 18 months (CI 95% 12-37). The median BFS was 73 months (CI 95% 48-NR). The median OS was 75 months (CI 95% 68-NR). The 5-year cumulative OS rate was 67% (95% CI, 53%-80%) (Figure 1). Twenty of 59 patients (34%) died during the study, and 17 died due to cerebral progression of lymphoma.
3.3.5 Treatment of the relapses
Twenty-seven of forty-two patients received only systemic treatment, 10/42 received combined treatment (local and systemic), and 4/42 did not receive any treatment. The treatment was unknown for one patient. Altogether, 14/42 (33%) patients received lenalidomide, 17/42 (40%) patients received ibrutinib, 18/42 (43%) patients received temozolomide and 11/42 (26%) patients received HD-MTX-based chemotherapy in the course of the disease. One patient received cerebral radiotherapy, and 11/42 (26%) patients received HCT-ASCT (seven for ocular relapse, two for cerebral relapse and two for ocular and cerebral relapse). Among them, seven patients were still in complete response at the time of analysis after a median follow-up for HCT-ASCT of 24 months, while four patients relapsed seven, 15, 21 and 39 months after HCT-ASCT, respectively. All patients with ocular relapse treated locally (6/42) relapsed and then received systemic treatment.
3.4 Prognostic factors
The main prognostic factors for OS are indicated in Table 3. Only age < 70 years and an HD-MTX dose ≥3 g/m2/injection were associated with better OS in both univariate and multivariate analyses, while KPS score, AH or vitreous IL-10 levels at baseline or type of HD-MTX based protocol were not associated with prognosis (Figure S1). OS after relapse was 29 months when the first relapse was cerebral (+/− ocular), while it was 55 months when the first relapse was only ocular (p < .001). Combination with local treatment was associated with a better OFS (55 vs. 18 months, p = .02) but did not change the OS, BFS or PFS (p = .2, p = .2 and p = .1, respectively). Levels of IL-10 in the vitreous or the aqueous humor at diagnosis did not predict ocular or CNS relapse. Unilateral vs bilateral ocular involvement had no significant impact in terms of BFS or OS. Both OS and BFS were comparable between the three treatment protocols (ie,(R)-MPV-(A), (R)-MBVP-(A) and (R)-MA), whereas OFS was better in the (R)-MBVP-(A) group compared to (R)-MPV-(A) and (R)-MA groups, (median OFS of 51 months vs. 24 and 9 months, respectively, p = .002). However, patients were younger in the (R)-MBVP-(A) group (median age of 59, 74 and 70 years, respectively, p = .004).
| N | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Median OS | p Value | Hazard ratio | p Value | ||
| Age | |||||
| ≥70 | 32 | 68 | .01* | 3.4 | .05* |
| <70 | 27 | NR | |||
| Sex | |||||
| Male | 14 | 74 | .3 | ||
| Female | 45 | 75 | |||
| Karnofsky performance status score | |||||
| ≤80 | 18 | 74 | .3 | 0.4 | .1 |
| >80 | 37 | 75 | |||
| Laterality of PVRL | |||||
| Unilateral | 20 | 51 | .08 | ||
| Bilateral | 37 | 75 | |||
| Hyalitis | |||||
| Yes | 50 | NR | .4 | ||
| No | 5 | NR | |||
| Best corrected visual acuity | |||||
| <3 | 9 | 74 | .2 | ||
| ≥3 | 43 | 75 | |||
| Meningeal disease | |||||
| Yes | 6 | NR | .1 | ||
| No | 38 | 75 | |||
| Chemotherapy protocols | |||||
| (Rituximab), methotrexate, etoposide, carmustin, prednisone, (cytarabine) ((R)-MBVP-(A)) | 35 | 75 | .1 | ||
| (Rituximab), methotrexate, vincristine, procarbazine, cytarabine ((R)-MPVA) | 11 | NR | |||
| (Rituximab), methotrexate, cytarabine | 11 | 45 | |||
| Dose of MTX per injection | |||||
| ≥3 g/m2 | 52 | 75 | .003* | 0.2 | .03* |
| <3 g/m2 | 7 | 30 | |||
| Rituximab | |||||
| Yes | 39 | 73 | .6 | ||
| No | 20 | 74 | |||
| Combined with local treatment | |||||
| Yes | 8 | 74 | .2 | ||
| No | 51 | NR | |||
- * means statistically significant.
- Abbreviations: MTX, methotrexate; OS, overall survival; PVRL, primary vitreoretinal lymphoma.
There was no difference between the long-term responder group (n = 16) and the non-responder group (n = 13) in terms of clinical and treatment characteristics.
4 DISCUSSION
To our knowledge, this study represents the largest series of isolated PVRL reporting the outcome of patients uniformly treated with IV HD-MTX based systemic therapy, as the first line of treatment according to the French national recommendations (Table S1). This series represents a real-life population of patients with isolated PVRL, as almost 90% of the patients with PVRL diagnosed between 2011 and 2018 in the LOC network received HD-MTX-based chemotherapy and were therefore included in the study. Chemotherapy protocols were similar to those used for PCNSL. Whereas the majority of the population was elderly (median age of 70 years), IV HD-MTX-based chemotherapy seemed to be feasible, with an acceptable level of toxicity. Most patients received ≥4 injections of MTX at a dose per injection ≥3 g/m2. No deaths or disruptions of therapy due to MTX toxicity were reported.
Despite a high percentage of patients with CR/uCR (70%), we report a high rate of ocular relapses (58%), compared to rates of 22% and 25% in the two largest series in the literature.8, 27 This high rate of ocular relapse probably explains why our median PFS is lower than that in most other series (18 months vs. 30-46 months in the literature).6, 7, 28, 29 This result might be due to the small percentage of patients with local treatment combined with systemic chemotherapy in our series. Some studies have suggested that although IV HD-MTX has good penetration through the blood-ocular barrier and the blood–brain barrier, the penetration of MTX in the two organs might still differ, and the maintenance of high levels of MTX in the vitreous might be more difficult by IV administration than by direct intravitreal injection.10, 30-32 Furthermore, in our series, patients who received both local and systemic treatments had better ocular-free survival (p = .02). The IV HD-MTX-based chemotherapy alone might not be sufficient to prevent ocular relapse. However, the rate of ocular relapse is quite variable in the literature. Recently, Ma et al. found an ocular relapse rate of 62% within 40 months of follow-up in 13 patients with isolated PVRL, although they received combined treatment with IV HD-MTX and intravitreal MTX.33 Castellino et al. reported an ocular relapse rate of 46% in a series of 33 PVRL patients comparing local, systemic and combined treatment.28
Another hypothesis for the high rate of ocular relapse in our series could result from the close systematic monitoring recommended by the LOC expert network. The AH IL-10 level appears to be a very valuable marker in the follow-up of the disease, especially when ocular relapse is not clinically obvious. Both IL-10 and IL-6 were the first cytokines shown to have proven value for the diagnosis of PVRL,24, 26 with a sensitivity and specificity of 0.78 and 0.97, respectively, for an IL-10 cutoff of 30 pg/ml in the AH25 and of 0.93 and 1.0, respectively, for an IL-10 cutoff of 65 pg/ml in the vitreous. The ISOLD score, based on the levels of IL10 and IL6 in the AH and vitreous, has shown a sensitivity and specificity of 0.93 and 0.95, respectively.34 However, the value of IL-10 and IL-6 levels after treatment and at relapse is not yet clearly defined in the literature. Very small series have described a decrease in IL-10 levels in the AH after intravitreal MTX or rituximab,32, 35, 36 but no data on IL-10 levels are available after chemotherapy. In our study, after first-line treatment, 87% of patients with a CR or uCR had undetectable IL-10 levels, whereas 92% of patients with SD/PD had detectable IL-10 levels. Compared to the gold-standard clinical IPCG criteria for defining a complete response, IL-10 levels were discordant in four patients. In three patients with a CR/uCR, IL-10 levels remained elevated, and two of them relapsed a few months after the end of the treatment. The third patient relapsed in the brain 1 year afterwards. Finally, one patient with PD had undetectable IL-10 levels, but IL-10 levels had never been elevated in his disease history, despite classic ophthalmological involvement with hyalitis. The IPCG criteria, based only on clinical issues, could therefore be easily met by the determination of IL-10 levels in the AH.
Despite a high rate of ocular relapse and a short PFS, the median OS reported in this study seems higher than what was previously reported in the large series for which the treatment was not uniform: 75 months in our series versus 34-58 months7, 8(Table S1). The first explanation could be that the systematic use of HD-MTX based systemic therapy as first-line treatment prevents brain relapse. The literature regarding the treatment of isolated PVRL, including almost exclusively retrospective studies, remains controversial regarding the use of local, systemic or local and systemic treatment. Regarding ocular radiotherapy, Mikami et al. and Teckie et al. found brain relapse rates of 55% and 42%, respectively, in patients treated with ocular radiotherapy after a median follow-up time of 36 and 25 months.18, 19 Berenbom et al. showed that the addition of chemotherapy to ocular radiotherapy delayed the onset of brain relapse in patients: the median BFS was 28 months with chemotherapy versus 8 months without (p = .24).15 Regarding intravitreal MTX, Akiyama et al. found a better two-year BFS with combined treatment (IV HD-MTX-based chemotherapy and intravitreal MTX) than with intravitreal MTX alone (58% vs. 38%) and a rate of brain relapse for the intravitreal MTX alone group of 88% at the 40-month follow-up.21 Hashida et al. found that prophylactic IV HD-MTX-based chemotherapy delayed the onset of cerebral involvement in a series of 26 patients with PVRL (43 months with IV MTX-based chemotherapy vs. 10 months with local treatment only (intravitreal injection of MTX or rituximab, p < .001).20
Three large retrospective studies,7, 8, 28 with 83, 78, and 33 patients with PVRL, respectively, compared systemic, local and combined treatment (Table S1). All three studies failed to show that systemic treatment was better than local treatment in terms of OS. However, the treatments received by the combined treatment group in those studies varied greatly and included chemotherapy and/or ocular radiotherapy and/or whole-brain radiotherapy and/or intrathecal chemotherapy and/or intravitreal MTX or rituximab. Furthermore, the proportions of patients receiving HD-MTX-based regimens among the three studies were not similar, with 70%, 40% and 30%, respectively.7, 8, 28 Our population was quite uniform in terms of treatment. Although OS was not different between treatment groups in the study by Castellino et al. the author found that PFS and BFS were better with combined treatment (p = .002 and p = .003).
Several studies with a smaller number of patients but uniformly receiving IV HD-MTX based systemic therapy showed encouraging results. Ma et al. and De la Fuente et al. described high rates of five-year overall survival of 68.8% and 80%, respectively, close to our series (66% at 5 years), using IV HD-MTX-based treatment combined with intravitreal MTX and ocular radiotherapy.33, 37 Kaburaki et al. found four-year BFS and OS of 90% and 89%, respectively, using IV HD-MTX based systemic therapy, intravitreal MTX and whole-brain radiotherapy in a small series of 11 patients with PVRL.38 Therefore, IV HD-MTX based systemic therapy might be efficient in preventing brain relapse and extending overall survival with or without the addition of local treatment.
The second explanation for the better OS in the present study could be the effectiveness of the treatment at relapse and the use of promising drugs in PVRL39-41: 89% of the patients received at least one systemic salvage therapy, including ibrutinib, temozolomide or lenalidomide, in 40%, 43% and 33% of cases, respectively, and 26% of patients received HCT-ASCT. Further studies are needed to study these first-line drugs as single agents or in combination with HD-MTX. The use of these strategies in first-line treatment in combination with HD-MTX might also be interesting.42
This work had several limitations, mainly due to the inherent biases of a retrospective study and to some missing data. Furthermore, the follow-up could be longer in this disease with prolonged survival. We only have one group of treatment, so it is difficult to measure the exact benefit of HD-MTX-based first-line treatment compared to local treatment. It is also difficult in such a retrospective study with heterogeneous HD-MTX based protocols to evaluate the impact of the drugs associated to MTX. Prospective and randomized studies would be very useful to progress in the management of PVRL but are very difficult to perform due to the rarity of the disease.
5 CONCLUSION
This “real-life” study shows that HD-MTX-based chemotherapy is feasible in PVRL, with an acceptable safety profile in an elderly population. Long-term BFS and OS rates were high, suggesting that this treatment strategy might be effective in preventing brain relapse. However, the rate of ocular relapse was disappointing. AH IL-10 levels should be included in the criteria for response and relapse for the early detection of ocular relapses.
In the future, the combination of HD-MTX based systemic therapy with local treatments or with drugs such as ibrutinib, lenalidomide or temozolomide might improve the prognosis of patients with PVRL. The role of intensive chemotherapy with autologous stem cell transplantation should also be addressed in this severe disease.
ACKNOWLEDGMENTS
We gratefully thank the patients and their families for their participation in this study. We acknowledge the research technicians of the LOC network (Bachir Aidoui, Diane Genet, Hassen Douzane, Yah-se Abada), all the members of the LOC network and the Institut National of Cancer (INCa).
CONFLICT OF INTEREST
S. Choquet declares conflict of interest with Roche, Janssen, Celgène, Abbvie, Sandoz, Biogaran and Accord Healthcare. L. Kodjikian declares conflict of interest with Abbvie, Allergan, Bayer, Novartis, Roche and Thea. P. Franciane declares conflicts of interest with Novartis. All other authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Institutional Ethical Committee of the coordinating center on April 24, 2018 and by the French “Commission Nationale de l'Informatique et des Libertés” (CNIL) (n°913170). This study was conducted in accordance with the Declaration of Helsinki.
PATIENT CONSENT
All patients gave informed consent for the submission of their data to the database.
Open Research
DATA AVAILABILITY STATEMENT
All the data are available in the national French database of the “Lymphome Oculo-Cérébral” (LOC) network.




