Iron and platelets: A subtle, under-recognized relationship
Abstract
The role of iron in the formation and functioning of erythrocytes, and to a lesser degree of white blood cells, is well established, but the relationship between iron and platelets is less documented. Physiologically, iron plays an important role in hematopoiesis, including thrombopoiesis; iron levels direct, together with genetic factors, the lineage commitment of megakaryocytic/erythroid progenitors toward either megakaryocyte or erythroid progenitors. Megakaryocytic iron contributes to cellular machinery, especially energy production in platelet mitochondria. Thrombocytosis, possibly favoring vascular thrombosis, is a classical feature observed with abnormally low total body iron stores (mainly due to blood losses or decreased duodenal iron intake), but thrombocytopenia can also occur in severe iron deficiency anemia. Iron sequestration, as seen in inflammatory conditions, can be associated with early thrombocytopenia due to platelet consumption and followed by reactive replenishment of the platelet pool with possibility of thrombocytosis. Iron overload of genetic origin (hemochromatosis), despite expected mitochondrial damage related to ferroptosis, has not been reported to cause thrombocytopenia (except in case of high degree of hepatic fibrosis), and iron-related alteration of platelet function is still a matter of debate. In acquired iron overload (of transfusional and/or dyserythropoiesis origin), quantitative or qualitative platelet changes are difficult to attribute to iron alone due to the interference of the underlying hematological conditions; likewise, hematological improvement, including increased blood platelet counts, observed under iron oral chelation is likely to reflect mechanisms other than the sole beneficial impact of iron depletion.
1 INTRODUCTION
The key role of iron in the production and function of red blood cells has been long established,1 but iron also plays a similarly important role for white blood cells.2 The present review aims to provide an update on the interactions between iron and platelets. These interactions, long observed by clinicians, have recently benefited from important advances that improve our understanding of their physiology and pathophysiology.
2 IRON AND PLATELETS: GENERAL ASPECTS
2.1 The platelet: an anucleated but sophisticated blood cell
2.1.1 Morphological and functional aspects
Platelets (or thrombocytes) are cytoplasmic fragments derived from bone marrow megakaryocytes and produced at a daily rate of approximately 1011.3,4 Once released into the plasma, they circulate, at a concentration of 150–400 × 109/L, and have a physiological lifespan of approximately seven to ten days. They undergo progressive senescence, particularly marked by desialylation of the platelet surface glycoproteins5 before being cleared by the macrophagic system, especially in the spleen and liver. Platelet life span is regulated by a finely tuned process, ensuring that 1011 platelets are removed daily.6 Platelet structure and composition reflect the numerous facets of their activities. They contain mitochondria, mRNA, and all the translational machinery needed for protein synthesis (approximately 5000 different proteins are present inside platelets7), including the ribosomes, endoplasmic reticulum, and Golgi apparatus. They host several types of storage granules containing a wide array of potentially active molecules (fibrinogen, growth factors, cytokines, chemokines, adenosine diphosphate [ADP], and serotonin),8 and they secrete a myriad of membrane vesicles (both exosomes and microvesicles) into the blood that together represent the majority of circulating vesicles.9 Moreover, platelets have adhesion and immune receptors.10, 11 They are also able to generate reactive oxygen species (ROS) especially as products of the electron transport chain. It is important to remember that ROS exert a physiologically pivotal role, as they fine-tune several signaling pathways.12-14
2.1.2 Pathophysiological aspects
Platelets are involved in both beneficial and deleterious processes. While the primary beneficial effect is their crucial function in hemostasis through clot formation, their appropriate positioning at the endothelial sites following vessel injury also puts platelets on the front line for sensing and counteracting pathogens. Thus, activated platelets are able to induce innate (natural) immunity15 by releasing antimicrobial proteins, chemokines and cytokines, and by recruiting neutrophils and monocytes/macrophages; platelets can also interact with the adaptive (acquired) immune system through crosstalks with B and T lymphocytes. From a detrimental point of view, hyperactivation of platelets can cause thrombus formation in atherosclerosis diseases or in inflammatory/infectious diseases,16 and excessive immune-related platelet activation can lead to thrombocytopenia as observed in autoimmune diseases (eg, systemic lupus erythematosus).17
2.2 Role of iron in platelet physiology
2.2.1 Role of iron in platelet formation
According to the classical view of hematopoiesis, thrombopoietin controls megakaryopoiesis and thrombopoiesis18 and platelets originate from bipotent megakaryocytic/erythroid progenitors (MEPs).4, 19 Several genetic or acquired factors, independent of thrombopoietin,20 direct the lineage commitment of MEPs toward either megakaryocyte progenitors (MkPs) or erythroid progenitors (ErPs). Genetic determinism of these processes has been suggested by genome-wide association studies that have identified specific polymorphisms that impact both red blood cell and platelet production.21, 22 Among acquired factors, iron status has recently been reported as critical for regulating the lineage commitment of MEPs. Using acquired (low iron diet) and genetic (Tmprss6−/−) mouse models of iron deficiency developing thrombocytosis, Xavier-Ferrucio et al.23, 24 demonstrated that lack of iron led to a preferential megakaryocytic commitment of MEPs. From a mechanistic viewpoint, the following characteristics were observed in MEPs: low intracellular labile iron, low ERK (extracellular signal-regulated kinase) signaling, and slow proliferation. Moreover, these findings were concordant with human primary MEPs in in vitro experiments: after they had been depleted of their iron sensor TFR2 (transferrin receptor 2), the cells recapitulated the megakaryocytic commitment bias observed in the above-mentioned Tmprss6−/− mouse model.23 Although cellular iron content of those human MEPs was not investigated, the authors considered that disrupting the TFR2 iron-sensing mechanism would mimic a low-iron environment. This may appear paradoxical because, in humans25 and mice,26 TFR2 loss of function causes marked systemic iron overload. Nevertheless, it has been shown that specific bone marrow TFR2 loss of function stimulates erythropoiesis without causing systemic body iron excess.27 Furthermore, using a systemic TFR2 deficient mouse model, Wortham et al. recently showed that extrahepatic (bone marrow and spleen) TFR2 deficiency was associated with increased erythropoiesis, independent of iron overload.28 The preferential orientation, in iron deficiency, toward the production of platelets instead of red blood cells remains to be explained. This regulatory mechanism may, on a chronic basis, preserve the use of iron for functions other than erythroid differentiation and, in acute clinical situations, help stop a life-threatening hemorrhage.24
2.2.2 Role of iron in mature platelets
Because platelets contain no nuclear genomic DNA, the major role of iron in DNA synthesis29 does not apply to them at the nuclear level, although it could play a role in mitochondrial DNA synthesis or repair (provided they occur). The lack of nucleus may have opened the window for improved platelet functional efficiency,30 and, given the role of iron in many cellular processes, significant functional interactions between iron and platelets would be expected (Figure 1).
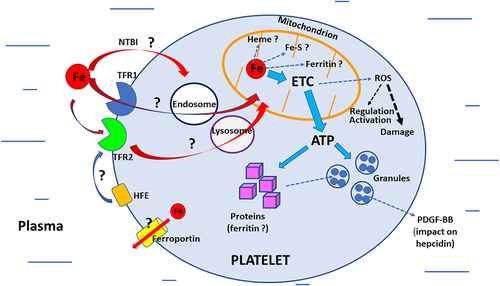
2.2.3 Iron and platelet mitochondria
The number of mitochondria in each thrombocyte is relatively low (less than ten). However, their functional efficiency at coping with intense activity requiring high energy (ATP) production is remarkable. This energy-consuming activity is notably related to granule secretion and platelet flexibility, that is, the capacity to change from a resting state to an activated one and vice versa. It has been shown that ATP production in platelet mitochondria is more efficient than ATP production in mitochondria of monocytes and lymphocytes.14, 31, 32 Knowing that ATP is derived from glycolysis and oxidative phosphorylation and that iron is involved in both processes33 throws light on interactions between iron and platelet mitochondria. Whether platelet mitochondria play a significant role in heme synthesis, iron–sulfur cluster biogenesis, or ferritin synthesis34, 35 remains to be explored; this is also the case for mitochondrial iron trafficking36 and any possible interactions between iron, platelet mitochondrial ferritin, and mitophagy.37
2.2.4 Iron trafficking and metabolism in platelets (other than mitochondria)
It is quite plausible that most platelet iron comes from the megakaryocytes themselves and that their original iron load is sufficient to meet the needs of the platelets during their short life. There are no reports, to our knowledge, documenting the presence of ferritin within the platelet cytosol, and data remains fragmentary on the role of potentially “active” iron actors at the platelet level itself. Thus, the presence of TFR1 (transferrin receptor 1), the cellular “entrance gate” for plasma transferrin-iron, is debated. Its expression was not found in human platelets by Hannuksela et al.,38 unlike the cellular iron sensor TFR2 or the HFE protein,38, 39 but it has been reported by the platelet proteome-data base.40 It remains to be shown whether classical TFR1-iron endocytosis41 is functional in platelets. If this is the case, it could open the possibility of a mechanism for iron delivery from the endosomes to the mitochondria either via a cytosolic pathway or, as proposed by Hamdi et al. in reticulocytes, through direct interaction between endosomes and mitochondria (the “kiss-and-run” hypothesis).42 It should be asked whether TFR2 could also be involved in iron entry into platelets and its delivery to mitochondria.41 The presence of non-transferrin bound iron transporters43 has not yet been confirmed. Ferroportin, the cellular iron exporter,44 has been detected in the platelet proteome-database,40 but its functional activity has not yet been documented. Although the expression of hepcidin, the iron hormone,45-47 has not been reported in platelets, it should be mentioned that the platelet-derived growth factor-BB (PDGF-BB), a potent angiogenic and chemoattractant agent48 produced by platelets (but also by other cell types), has been shown to mediate hypoxia-induced downregulation of hepcidin in hepatocytes, increasing the availability of plasma iron for erythropoiesis.49
3 IRON AND PLATELETS: CLINICAL AND MECHANISTIC ASPECTS, IRON DEFICIENCY AND PLATELETS
3.1 Impact of iron deficiency on platelets
Table 1 presents the different effects of iron deficiency on platelets.
| Absolute ID (decreased total body iron stores) | Functional ID (inflammation-related iron misdistribution) | Iron overload (increased total body iron stores) | ||
| Genetic (hemochromatosis) | Acquired (thalassemia/MDS) | |||
| Thrombocytosis | ++ | ++ | Not documented | 
|
| Thrombocytopenia | + (severe IDA) | ++ (acute inflammation) | Not documented (except if cirrhosis) | |
| Thrombopathy | + | + | Unlikely | |
3.1.1 Absolute iron deficiency
Absolute iron deficiency may be defined as a decrease in total body iron stores and can lead to iron deficiency anemia (IDA). The main causes of absolute iron deficiency are iron losses of gynecological (menorrhagia), digestive (peptic ulcer, colon cancer), blood donation origin,50 decreased duodenal iron intake51, 52 due to an iron-poor diet, coeliac disease, long term use of proton pump inhibitors, or iron refractory iron deficiency anemia (IRIDA).53
Thrombocytosis (platelet count above 400 × 109) is a classical clinical feature in chronic iron deficiency, described as one of the conditions of” reactive” thrombocytosis.54 If we consider only absolute iron deficiency (i.e., excluding iron deficiency related to inflammation, see section 3.1.2), reactive thrombocytosis has been observed at the following frequencies: 13% in a study of 615 subjects by Kuku et al.,55 28% in 86 women by Kadikoylu et al.,56 and almost 33% in a large population of 36 327 IDA patients by Song et al.57 As mentioned above, the main mechanism whereby absolute iron deficiency favors thrombocytosis could be a shift of MEPs toward MkPs at the expense of ErPs.23 It has also been reported that thrombocytosis in IDA could involve a downregulation of tubulin, which is one of the main components of the platelet cytoskeleton,58 but this may be a consequence rather than a causative factor. Estimates of the thrombotic risk in the event of thrombocytosis related to absolute iron deficiency differ greatly: no cases of thrombosis were recorded by Kuku et al.,55 whereas thrombotic complications were observed by Song et al. in 16% of IDA patients with thrombocytosis, compared with 8% in IDA without thrombocytosis.57 Recently, Jimenez et al.59 provided original experimental mechanistic data on the relationship between decreased body iron stores and the development of thrombosis. Using rat models of dietary ID, they showed that ID was an independent risk factor for the development of venous or arterial thrombosis. Hypercoagulability was found, together with increased platelet and plasma P-selectin (reflecting hyperactivity), and with impaired platelet adhesion and aggregation under shear flow; importantly, all these changes were reversed by iron supplementation. It should be noticed that the relationship between iron deficiency and venous thromboembolism (VTE), which was confirmed by a broad-based-population study,60 might involve other factors than thrombocytosis. Thus, still in the field of iron metabolism, transferrin upregulation could play a significant role, as shown by Tang et al.61 These authors found, both in vitro and in vivo, that ID, estrogen administration, and exogenous transferrin or transferrin overexpression induced hypercoagulability and aggravated ischemic stroke. In contrast, anti-transferrin antibodies, transferrin knock-down, and designed peptide inhibitors exerted anti-ischemic stroke effects. However, contrary to the view that ID may explain the association between red cell distribution width and risk of VTE, Ellingsen et al.62 reported that increased plasma levels of hepcidin, considered as biomarkers of iron stores, were associated with an increased risk of VTE. It should be noted, however, that variations of both hepcidin and ferritin levels remained minimal.
Thrombocytopenia can be observed in severe iron deficiency, and clinicians should be aware of this rare event (less than 50 reported cases55, 63-66). It can be falsely diagnosed as an immune thrombocytopenia that can be associated with iron deficiency secondary to bleeding.67 Two factors may favor the diagnosis of iron deficiency related thrombocytopenia: (i) the severity of iron deficiency with marked decreases in hemoglobin, MCV (erythrocyte mean corpuscular volume), and ferritin values; and (ii) a low percentage of reticulated platelets (or low immature platelet fraction) (IPF), which orientates toward a central thrombocytopenia (a state of impaired platelet production, as opposed to the increased destruction by autoimmune attack as occurring in immune thrombocytopenia).66, 68 Moreover, IPF increases under iron supplementation in parallel with reticulocytes and platelet counts.66 The mechanism whereby major ID, in contrast to minor ID, causes thrombocytopenia remains to be specifically identified, but it is likely that, in this severe clinical situation, the erythropoiesis pathway becomes vital and is therefore “preferred” to thrombopoiesis.
3.1.2 Functional iron deficiency
Functional iron deficiency occurs mainly in inflammatory situations in which systemic iron distribution is modified but total body iron stores remain unchanged. Indeed, especially under stimulation of the IL6-STAT3 signaling pathway, hepatocyte hepcidin secretion is increased, leading to low duodenal iron absorption and low splenic iron release, both of which are factors causing low plasma iron and low plasma transferrin saturation.
As a result, iron entry into the bone marrow and erythrocyte production decreases, contributing, in the long-term, to the development of anemia of chronic disease. Importantly, the decreased plasma iron compartment is associated with an increased macrophagic iron compartment. This macrophagic iron retention (bone marrow, spleen, liver) is related to decreased cellular iron release due to the decrease of ferroportin iron export activity resulting from high hepcidin levels.69-72 On the whole, this creates iron misdistribution with normal overall body iron stores.
From the platelet perspective, the links between inflammation and iron may have several important aspects. On the one hand, it is expected that decreased iron availability to hematopoietic precursors (due to low iron-saturation of transferrin and/or to hepcidin-mediated iron-trapping in bone marrow macrophages) may contribute to thrombocytosis. As to the development of ID-related thrombocytopenia, it is less likely due to less potential ID severity during inflammation than in absolute ID. On the other hand, as mentioned above, inflammation itself can interact with platelets independently of iron. This is especially observed in acute inflammation.
During infection, thrombocytopenia occurs rapidly due to the extensive platelet consumption that follows the release of immunomodulatory agents and the interaction of platelets with neutrophils to form neutrophil extracellular traps.73 This process may have severe clinical consequences, including increased mortality from loss of vascular integrity, hemorrhage, and even septic shock.74 It is followed by a quick regeneration of platelets to counteract inflammation-induced thrombocytopenia. Haas et al. have shown that inflammation drives efficient cell cycle activation and maturation of stem-like megakaryocyte committed progenitors that rapidly replenishes the platelet pool during acute inflammation.75 Thrombopoietin may be involved in this thrombopoiesis activation mechanism through its stimulation by IL-6.76
Clinically, inflammation-related thrombocytosis is observed in infectious diseases77 but also in non-infectious ones such as inflammatory bowel disease,78, 79 where a correlation has been found between plasma hepcidin levels and platelet counts,80 auto-immune disorders (systemic lupus erythematosus,81 juvenile arthritis82), various malignancies including solid tumors,83 or chronic myeloproliferative neoplasms,84 in which thrombocytosis can also, of course, be of primary origin. As for the thrombotic risk, it may result from the conjunction of reactive thrombocytosis and the natural history of the causal disease.85
3.1.3 Impact of platelet alterations on iron metabolism
Whether of acquired or genetic origin, severe thrombocytopenia or thrombopathy (functional platelet alterations) can lead to hemorrhage and in turn to absolute iron deficiency. Moreover, depending on the etiology, inflammation may also interfere with iron metabolism by favoring systemic iron misdistribution. It should be noted that platelet counts alone are a poor predictor of hemorrhagic risk, unless platelet counts are ≤5 x 109/L, as shown in patients with hypoproliferative thrombocytopenia due to stem cell transplants or chemotherapy for malignancy.86
3.2 Iron overload and platelets
Table 1 presents the different effects of iron overload on platelets.
3.2.1 Genetic iron overload (hemochromatosis) and platelets
There are no available data documenting the potential impact of hemochromatosis on blood platelet counts,87, 88 unless there is a high degree of hepatic fibrosis,89 possibly related to hypersplenism (portal hypertension) and /or hepatocellular failure (deficient synthesis of thrombopoietin). It should be recalled, however, that in hemochromatosis, such severe hepatic dysfunction is mostly observed when there are cofactors of liver toxicity (e.g., alcohol, non-alcoholic fatty liver disease). Regarding platelet quality, Mikaelsdottir et al.90 studied platelet-rich plasma obtained from 10 newly diagnosed hemochromatosis patients undergoing phlebotomy therapy over a seven-day storage period. They concluded that no differences were observed between the patients and a group of healthy donors, for platelet aggregation (after activation with ADP, arachidonic acid, collagen, or epinephrine), as well as for expression of surface (CD62P and CD42B) and secreted (sCD62P and sCD40L) activation markers. These results differ from previous data showing that iron levels found in hemochromatosis patients inhibited ɣ-thrombin-induced platelet aggregation.91 Moreover, a recent study92 of 43 iron overloaded patients, including 19 C282Y/C282Y hemochromatosis patients and one case of HJV (hemojuvelin) hemochromatosis, reported that plasma transferrin saturation was inversely correlated with platelet reactivity. Iron overload has also been shown to alter mitochondrial respiration in a rat model of hemochromatosis,93 and morphological alterations of hepatocyte mitochondria have been observed in severe cases of human hemochromatosis.94 Moreover, focusing on human platelets and iron exposure, non-heme iron was unable to induce platelet activation, unless platelets had been first primed with collagen, in which case total platelet aggregation occurred.95 Hemin induced platelet activation and death through ferroptosis,96 involving the C-type-lectin-like receptor-2.97 It is therefore possible that human platelet mitochondria also represent a target of iron-related ferroptosis in hemochromatosis.
3.2.2 Acquired iron overload and platelets
Chronic acquired iron overload is mainly the result of multiple transfusions and/or ineffective erythropoiesis as occurs in ß-thalassemias98, 99 and myelodysplastic syndrome (MDS)100 or following therapeutic ablation in bone marrow transplantation.2 Due to platelet changes, whether quantitative101-103 and/or qualitative,99, 104-106 which can develop as part of the underlying hematological diseases (or as a result of their medications), it is difficult to attribute platelet modifications to iron overload alone. One way to approach this issue is to evaluate the impact of iron chelation treatment on thrombocytes. Numerous studies have reported hematological improvement, including platelet counts, in MDS patients under oral chelation by deferasirox.107-110 However, most of these studies did not find any significant correlation with decreased body iron stores; only Cheong et al reported, in a prospective, multi-center, open-label study of 96 patients, that elevated platelet counts (together with increased hemoglobin) were associated with a significant decrease of both serum ferritin and liver iron concentration.107 These results have led to a search for mechanisms other than the iron factor alone that could account for hematological improvement. In vitro studies have identified the impact of deferasirox on ROS,111 NfκB,112 and mTOR.113 Performing genome-wide transcriptomics in peripheral blood mononuclear cells of 15 patients with low-grade MDS treated with deferasirox, Sanchez et al114 identified deregulated genes likely to contribute to hematological improvement: downregulation of genes related to the NfκB pathway, downregulation of ɣ-interferon, inhibition of NFE2L2/NRF2 that plays a key role in reducing ROS, overexpression of miR-125b (micro-RNA acting as a key regulator of hematopoietic stem cells), and overexpression of GFII (gene growth factor independent I), which is a transcriptional repressor playing a critical role in hematopoiesis.
4 CONCLUSION
Iron is required for the overall process of hematopoiesis, and fluctuations of the bone marrow iron load environment affect whether cells become platelets or erythrocytes. A low iron context favors platelet synthesis. Iron is also involved in the mitochondrion machinery that is particularly efficient in platelets. Absolute iron deficiency is frequently associated with thrombocytosis, which may increase the risk of vascular thrombosis. Thrombocytopenia can also occur in severe iron deficiency. Functional iron deficiency (misdistribution) develops during inflammatory conditions and may be associated with early thrombocytopenia, followed by rapid replenishment of the blood platelet pool, with sometimes reactive thrombocytosis. Whether chronic iron overload in hemochromatosis can alter platelet functioning with clinical consequences remains to be explored. Oral iron chelation improves platelet blood count in acquired (transfusional and/or dyserythropoietic) iron overload, but without strong evidence of the role of iron depletion itself. The main mechanistic explanation is likely the disruption of several signaling pathways by the iron chelator. In order to better understand the clinical interactions between iron and platelets, further basic research should focus on the putative presence and role of the various actors of cellular iron transport and metabolism in platelets, keeping in mind that platelet iron may originate from the iron contained in their mother cells.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable - no new data generated




