Haploidentical vs matched unrelated donor transplantation for acute myeloid leukemia in remission: A prospective comparative study
Funding information: Ministry of Health & Welfare, Republic of Korea, Grant/Award Number: HI18C0480; Korea Health Industry Development Institute (KHIDI); National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea, Grant/Award Number: HA17C0042
Abstract
Despite comparable outcomes of haploidentical transplants (Haplo-HSCT) with HLA-matched unrelated transplants (MUD-HSCT) in retrospective comparisons, few studies have prospectively compared Haplo-HSCT with MUD-HSCT in AML. Here, we prospectively compared the outcomes of Haplo-HSCT with MUD-HSCT for AML in remission (n = 110) to prove non-inferiority of overall survival in Haplo-HSCT. Both groups were well balanced in factors related to biological features of AML and measurable residual disease (MRD) status by Wilms' tumor gene 1 (WT1) assay. A unique, reduced-toxicity preparative regimen was used for Haplo-HSCT, whereas mostly-myeloablative regimen was for MUD-HSCT. Both groups showed similar patterns of neutrophil and platelet recovery, whereas delayed T-cell reconstitution in Haplo-HSCT was found compared with MUD-HSCT. No significant differences were found in acute or chronic graft-vs-host-disease (GVHD) and post-transplant infectious events with an exception of EBV or CMV infection, which occurred more frequently in Haplo-HSCT. After a median follow-up of 47 months, no significant differences in overall survival (65% vs 54%, P = .146), disease-free survival (67% vs 53%, P = .142), relapse (20% vs 21%, P = .858), non-relapse mortality (14% vs 26%, P = .103), or GVHD-free/relapse-free survival (54% vs 41%, P = .138) were observed for Haplo-HSCT vs MUD-HSCT. In multivariate analysis, WT1 expression before transplantation independently predicted relapse, resulting in inferior survival. Separate analysis of unenrolled patients (n = 110) who were excluded or refused to participate in this study showed consistent results with enrolled patients. This prospective study demonstrated the non-inferiority of Haplo-HSCT to MUD-HSCT for AML in remission, and validated the role of WT1 quantification as an MRD marker (ClinicalTrial.gov identifier: NCT01751997).
1 INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment option for patients with acute myeloid leukemia (AML) in complete remission (CR).1 Human leukocyte antigens (HLA)-matched sibling donor (MSD) is considered the primary choice for optimal transplantation with acceptable survival outcomes.1 If no appropriate MSD is available, alternative donors (including unrelated, haploidentical, or cord blood) could be used for AML, with high-risk features mainly based on cytogenetic and/or molecular abnormalities.1 Among alternative donors, recent data from large registries has confirmed the rapid increase in the number of haploidentical transplants (Haplo-HSCT),2, 3 due to the potential benefit associated with immediate donor availability and the widespread use of T-cell-replete platforms developed worldwide.4
Drawbacks of Haplo-HSCT, such as very strong host-vs-graft and graft-vs-host alloresponses, were initially overcome through transplantation of a “mega-dose” of T-cell-depleted peripheral blood stem cells (PBSC) and no post-transplant pharmacologic immunosuppression.5-7 The use of profound T-cell-depleted platforms has been rather unsatisfactory, due to deep and prolonged immunosuppression responsible for infectious complications, leading to higher non-relapse mortality (NRM) despite undergoing the inconvenience of cell manipulation.6 More recent transplantation protocols using T-cell-replete grafts combined with enhanced post-transplant immunosuppression showed favorable outcomes and substantially extended the use of Haplo-HSCT.4, 7 The feasibility of T-cell-replete Haplo-HSCT has now been established in several retrospective and a few prospective studies that included thousands of patients, but prospective comparisons are unavailable.4 Indeed, it is necessary to address the efficiency of Haplo-HSCT by prospective comparisons with other graft sources in well-selected AML populations characterized by their cytogenetic or molecular risk profile.4
We developed a protocol for Haplo-HSCT using T-cell-replete PBSC and a unique conditioning regimen consisting of fractionated total body irradiation (TBI) of 800 cGy, fludarabine, and busulfex with standard graft-vs-host disease (GVHD) prophylaxis supplemented by antithymocyte globulin (ATG). The feasibility of Haplo-HSCT using our unique, reduced-toxicity conditioning (RTC) regimen with T-cell-replete PBSC was reported previously with excellent engraftment and favorable survival outcomes.8, 9 To evaluate the efficacy of our Haplo-HSCT protocol, the current study prospectively compared the clinical features and outcomes of Haplo-HSCT with HLA-matched unrelated transplants (MUD-HSCT) based on the hypothesis that the 3-year overall survival (OS) after Haplo-HSCT is not inferior to OS after MUD-HSCT. In addition, this prospective study evaluated predictive role of pre-transplant Wilms' tumor gene 1 (WT1) measurable residual disease assay because of limited use in practice due to the lack of prospective study despite the promising role in previous reports.10, 11
2 METHODS
2.1 Study designs
In this prospective, biologically randomized, single center, open-label study, patients at CR before transplantation were firstly assigned to MUD-HSCT in the absence of MSD and secondly to Haplo-HSCT in the absence of MSD and MUD if they met the eligibility criteria. Patients were eligible for study participation if they were 18 to 65 years of age; had ECOG performance score <2; had unfavorable prognostic factors, including intermediate or high-risk features based on the 2012 National Comprehensive Cancer Network (NCCN) criteria;12 had prior hematological malignancies; or presented a leukocyte count higher than 100 000/μL in first or second CR. All donors were matched by high-resolution DNA matching for HLA-A, HLA-B, HLA-C, and HLA-DR alleles. Unrelated donor search was conducted during consolidation chemotherapies through two nation-wide registries in Korea before assigning the patient to a haploidentical donor. It took about 6 weeks to confirm the unavailability of matched unrelated donors or refusals of potential candidates. Diagnostic bone marrow (BM) samples obtained from all patients were analyzed for mutations involving NPM1, FLT3, CEBPA, and KIT using well-established protocols.13 The current study commenced in January of 2013 and patient accrual was completed in May of 2018. The Institutional Review Board of the Catholic Medical Center approved the current study. All analyses were performed according to the Institutional Review Board guidelines and the tenets of the Declaration of Helsinki.
2.2 Transplant procedures
Conditioning regimens consisted of myeloablative (MAC; cyclophosphamide 120 mg/kg combined with 1320 cGy of fractionated TBI or busulfex [12.8 mg/kg]) or reduced-intensity regimen (RIC; busulfex [6.4 mg/kg] and fludarabine [150 mg/m2] with 400 cGy of fractionated TBI) for MUD-HSCT14 and reduced-toxicity conditioning (RTC; busulfex [6.4 mg/kg] and fludarabine [150 mg/m2] with 800 cGy of fractionated TBI) for Haplo-HSCT.9 Rabbit ATG (Thymoglobulin, Sanofi/Genzyme, Cambridge, MA) was administered at a dose of 1.25 mg/kg/day on days −3 to −2 for MUD-HSCT and days −4 to −1 for Haplo-HSCT. T-cell-replete PBSC were infused and identical GVHD prophylaxis was performed using tacrolimus and a short course of methotrexate.9, 11, 14 Treatment courses and transplantation procedures, including serial chimerism of donor and recipient cells and immune reconstitution in the peripheral blood (PB), and WT1 quantification in BM were performed as previously described.8, 11
2.3 Study end points
The primary end point of the study was OS at 3 years. Secondary end points included engraftment, immune reconstitution, cumulative incidence of acute and chronic GVHD, cytomegalovirus (CMV) infection, and other infectious complications. Other survival end points, including cumulative incidence of relapse (CIR) and non-relapse mortality (NRM), disease-free survival (DFS), and a composite end point of GVHD-free and relapse-free survival (GRFS)15 and the role of MRD assessment with WT1 quantification were also explored. This study was registered at Clinical trial.gov (NCT01751997).
2.4 Definitions and statistical analysis
Neutrophil and platelet engraftment were defined as an absolute neutrophil count of >0.5 × 109/L during the first of 3 consecutive days, or a platelet count of >20 × 109/L without transfusion support during the first of 7 consecutive days. Acute and chronic GVHD, CMV infection, sinusoidal obstruction syndrome, thrombotic microanigopathy, or hemorrhagic cystitis were diagnosed and graded as described in our previous study.8
To calculate the sample size, we assumed that (a) the 3-year OS rate of Haplo-HSCT is not inferior to that of MUD-HSCT, and (b) referenced data of 3-year OS rate of MUD-HSCT is 44% (95% CI: 41-47), which was derived from an Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation.16 An acceptable noninferiority margin was defined 41% that preserves a minimum clinically acceptable 3-year OS rate of MUD-HSCT. The expected 3-year OS rate of MUD-HSCT in our center was 64%. Considering the expected 3-year OS rate of Haplo-HSCT will be 64%, we calculated the absolute difference (23%, 3-year OS rate in our center of 64%—the lower bound of the confidence interval of the reference registry data). We estimated sample size of 110 patients (55 patients in each group) calculated by non-inferiority log-rank test analysis. Assuming 5% are lost to follow-up, the planned number of enrolled patients was 116 patients. Confirmatory testing of the pre-specified primary outcome measure, 3-year OS, was performed in the full analysis set at a type 1 error level of 0.5 (two-sided). All the P-values are considered descriptively (including those that are not significant).
Categorical variables were compared using chi-squared test or Fisher's exact test, while continuous variables were analyzed with Student's t-test or Wilcoxon's rank-sum test. OS, DFS, and GRFS curves were plotted using the Kaplan-Meier method and analyzed with the log-rank test. Multivariate analysis for OS, DFS, and GRFS included variables with a P-value <.10, as determined by univariate analysis, which were considered for entry into the model selection procedure based on the Cox proportional hazards model. The cumulative incidence was used to estimate the probability of CIR, NRM, each subtype of GVHD, and CMV infection, and compared using the Gray test. CIR and NRM were competing events for each other. In other complications, deaths before the occurrence of each complication were treated as competing events. Multivariate analysis for CIR, NRM, each subtype of GVHD, and CMV infection included variables with a P-value < .10, as determined by univariate analysis, which were considered for entry into the model selection procedure based on a proportional hazards model for a sub-distribution of the competing risk factors. Statistical significance was determined as a P-value ≤.05 (two-tailed). All statistics were conducted using SPSS, version 13.0 (SPSS, Inc., Chicago, IL) and R-software (version 3.4.1, R Foundation for Statistical Computing, 2017).
3 RESULTS
3.1 Patient characteristics
Fifty-five patients were enrolled for each arm (Supplementary Figure S1). Until the last patient's accrual at May 2018 to Haplo-HSCT, a total of 220 patients met the study eligibility criteria and 110 patients were excluded or refused to participate in this study (Supplementary Table S1). Most reasons for refusals were fear or repulsion about more frequent sampling of blood or BM necessitated by participation in the study. The accrual for MUD-HSCT was finished 2 months earlier than Haplo-HSCT. Three more patients who underwent MUD-HSCT until last accrual for Haplo-HSCT were included in non-enrolled group. Another 110 patients who agreed to be enrolled were prospectively evaluated (Supplementary Figure S1). The donor assignment pattern between groups was parallel during the study period (Supplementary Figure S2). There was no significant difference between enrolled and non-enrolled patients with the exception of more advanced disease status at transplantation and a higher number of infused CD34+ cells in non-enrolled patients (Supplementary Table S2). Demographic characteristics of the enrolled patients are summarized in Table 1. Fifty-five patients in each group underwent MUD-HSCT and Haplo-HSCT, and the median age of patients was higher in Haplo-HSCT than in MUD-HSCT, but both groups had similar comorbidity index before transplantation. Otherwise, the two groups were well matched in terms of disease status and MRD status by WT1 levels before transplantation; AML-related factors, including white blood cell (WBC) counts; AML type; cytogenetic or molecular features; and donor-related factors, including donor age, sex match, or CMV match. All patients received PBSC from fully 8/8 HLA allele matched donors in the MUD-HSCT cohort or a direct family members who had more than 4 out of 8 allele mismatched in the Haplo-HSCT cohort. The conditioning regimen for MUD-HSCT was determined by age and/or comorbidity, consisting of 86% of MAC and 14% of RIC, while all patients in the Haplo-HSCT cohort received the same RTC. There was no significant difference in the number of CD34+ cells in stem cells.
| MUD-HSCT (n = 55) | Haplo-HSCT (n = 55) | P value | |
|---|---|---|---|
| Median age, y (range) | 41 (18-65) | 48 (18–65) | .008 |
| Age, n (%) | .013 | ||
| <60 years | 52 (95) | 42 (76) | |
| ≥60 years | 3 (5) | 13 (27) | |
| Sex, n (%) | .699 | ||
| Male | 33 (60) | 31 (56) | |
| Female | 22 (40) | 24 (44) | |
| White blood cell count per liter at diagnosis, n (%) | 11.8 (0.45-162.0) | 6.1 (0.4-266.2) | .856 |
| <100 | 51 (93) | 50 (91) | 1.000 |
| ≥100 | 4 (7) | 5 (9) | |
| AML type, no (%) | .822 | ||
| De novo | 46 (84) | 49 (89) | |
| Secondary | 8 (14) | 5 (9) | |
| Therapy-related | 1 (2) | 1 (2) | |
| Cytogenetic risk by MRC at diagnosis, n (%)a | .841 | ||
| Favorable | 6 (11) | 8 (14) | |
| Intermediate | 38 (69) | 37 (67) | |
| Adverse | 11 (20) | 10 (18) | |
| 2012 NCCN risk classification at diagnosis, n (%)b | .580 | ||
| Favorable | 3 (5) | 6 (11) | |
| Intermediate | 34 (62) | 32 (58) | |
| Adverse | 18 (33) | 17 (31) | |
| Mutations at diagnosis, nc | |||
| FLT3-ITD | 6 | 9 | |
| FLT3 Tyrosine kinase domain | 4 | 3 | |
| NPM1 | 5 | 10 | |
| CEBPA | |||
| Mono-allelic | 8 | 6 | |
| Bi-allelic | 4 | 1 | |
| KIT | 7 | 8 | |
| AML disease status at transplantation, n (%) | .320 | ||
| CR1 | 52 (95) | 48 (87) | |
| CR2 | 3 (5) | 7 (13) | |
| WT1 levels at transplantation, n (%)d | .614 | ||
| <250 copies | 37 (88) | 32 (84) | |
| ≥250 copies | 5 (12) | 6 (16) | |
| Median interval between diagnosis and transplantation, days (range) | 199 (107-302) | 197 (87-302) | .180 |
| Medina donor age, years (range) | 29 (19-46) | 28 (6-66) | .583 |
| Donor age, n (%) | .489 | ||
| <40 years | 52 (95) | 49 (89) | |
| ≥40 years | 3 (5) | 6 (11) | |
| Donor relationship, n | |||
| Unrelated | 55 | ||
| Haploidentical family (Donor – Recipient) | 55 | ||
| Offspring - Parents | 44 | ||
| Donor age ≥ 20 years | 31 | ||
| Donor age 10 ~ 19 years | 9 | ||
| Donor age <10 years | 4 | ||
| Sibling - Sibling | 7 | ||
| Parents - Offspring | 4 | ||
| Sex match, n (%) | .207 | ||
| Female to male | 7 (13) | 12 (22) | |
| Others | 48 (87) | 43 (78) | |
| CMV match, recipients/donors, n (%) | .321 | ||
| +/+ | 50 (91) | 46 (84) | |
| +/− | 4 (7) | 8 (14) | |
| −/+ | 1 (2) | 0 | |
| −/− | 0 | 1 (2) | |
| HCT-CI, median (range) | 2 (0–5) | 2 (0–9) | .723 |
| Conditioning regimens, n (%) | <.001 | ||
| Myeloablative | |||
| CY + TBI | 35 (64) | ||
| CY + BU | 12 (22) | ||
| Reduced-toxicity | |||
| FLU+BU + TBI (800 cGy) | 55 (100) | ||
| Reduced-intensity | |||
| FLU+BU + TBI (400 cGy) | 8 (14) | ||
| ATG, total dose | <.001 | ||
| 2.5 mg/kg | 55 (100) | ||
| 5.0 mg/kg | 55 (100) | ||
| Matched HLA loci | <.001 | ||
| 4/8 | 40 (73) | ||
| 5/8 | 15 (27) | ||
| 6/8 | 0 | ||
| 7/8 | 0 | ||
| 8/8 | 55 (100) | 0 | |
| Number of transplantation, n (%) | .438 | ||
| First | 53 (96) | 50 (91) | |
| Second | 2 (4) | 5 (9) | |
| Median CD34+ count, ×108/kg (range) | 5.11 (1.2-16.97) | 6.46 (1.37-19.37) | .131 |
- Abbreviations: AML, acute myeloid leukemia; ATG, antithymocyte globulin; BU, busulfex; CMV, cytomegalovirus; CR, complete remission; CY, cyclophosphamide; −host disease; ELN, EuropeanLeukemia Net; FLU, fludarabine; HCT-CI, hematopoietic stem cell transplantation-comorbidity index; Haplo-HSCT, haploidentical transplant; MRC, Medical Research Council; MUD-HSCT, matched unrelated transplant; n, number; NCCN, national comprehensive cancer network; TBI, total body irradiation; WT1, Wilms' tumor gene 1.
- a Cytogenetic risk group was defined by refinement of cytogenetic classification by the United Kingdom Medical Research Council trials.17
- b Risk stratification was defined by 2012 NCCN criteria.12
- c Diagnostic BM samples obtained from all patients were analyzed for mutations involving NPM1, FLT3, CEBPA and KIT using well established protocols.13, 14, 18
- d The levels of WT1 in samples from bone marrow (BM) at diagnosis and pre-transplantation were determined by real-time quantitative PCR (RQ-PCR) using WT1 ProfileQuant kit (Ipsogen, Marseille, France). WT1 gene transcripts generated by RQ-PCR were normalized with respect to the number of ABL1 transcripts and expressed as copy numbers per 104 copies of ABL1.11 AMong 110 patients, 18 patients who had lower expression of WT1 less than 250 copies at diagnosis and 12 patients who were not available for BM samples at diagnosis were excluded. Finally, 80 patients were evaluated for WT1 levels before transplantation.
3.2 Engraftment and immune reconstitution
The median time for neutrophil and platelet engraftment was not significantly different between MUD-HSCT and Haplo-HSCT (Supplementary Figure S3). After myeloid recovery, all patients achieved sustained, full donor chimerism (mean 99.4%, range 97%-100%) by day 30 after allo-HSCT without difference between groups (P = .247). No recipient experienced secondary engraftment failure.
Post-transplantation immune reconstitution in PB was analyzed in 42 of the MUD-HSCT cohort and 44 of the Haplo-HSCT cohort who survived at least 6 months without relapse between 1 month and 12 months after transplant (Supplementary Figures S4A-E). The two groups showed different patterns of immune reconstitution. Haplo-HSCT had significantly lower number of T-cells, including CD4 and CD8 positive subsets, at a month after transplant than did MUD-HSCT, whereas higher number of B and NK cells were reported in Haplo-HSCT within 6 months after transplant.
3.3 GVHD and CMV infection
There were no significant differences in the cumulative incidence of acute GVHD (P = .103; Supplementary Figure S5A) between MUD-HSCT and Haplo-HSCT. The cumulative incidence of grades II to IV acute GVHD for MUD-HSCT and Haplo-HSCT were 33% and 24%, respectively (P = .266; Supplementary Figure S5B). No significant differences in grades III and IV acute GVHD were detected between the two groups (9% for MUD-HSCT vs 7% for Haplo-HSCT; P = .761). The cumulative incidence of chronic GVHD in MUD-HSCT and Haplo-HSCT was 49% and 50%, respectively, without significant difference (P = .678; Supplementary Figure S5C). The proportion of moderate and severe chronic GVHD did not differ significantly among the groups (24% for MUD-HSCT vs 15% for Haplo-HSCT, P = .251; Supplementary Figure S5D).
Seventy-four patients had tested positive in at least one polymerase chain reaction (PCR) assay for CMV DNA at a median of 27 days (range 12-157) after allo-HSCT, and 41 patients (37%) required preemptive treatment with ganciclovir or foscarnet. Eighteen patients developed CMV disease, including CMV enterocolitis (colon, n = 10; duodenum, n = 2; stomach, n = 2), CMV pneumonia (n = 2), and CMV retinitis (n = 1). Patients with CMV infection had a significantly lower number of T-cells, including CD4 and CD8 positive subsets, at a month after transplant compared with patients without CMV infections (Supplementary Figures S4F-J). The cumulative incidence of CMV DNAemia (Supplementary Figure S5E), therapy-required CMV infection (Supplementary Figure S5F), and CMV disease (Supplementary Figure S5G) were significantly higher in Haplo-HSCT than in MUD-HSCT (84% vs 51%, P < .001; 49% vs 26%, P = .003; 26% vs 7%, P = .008), which was also confirmed in multivariate analysis (Supplementary Table S3).
3.4 Other complications
Epstein-Barr virus (EBV) reactivation was more frequent in Haplo-HSCT than in MUD-HSCT (49% vs18%, P = .001), but no patients developed post-transplantation lymphoproliferative disease in either group. There were no differences between MUD-HSCT and Haplo-HSCT in terms of other infectious complications (Supplementary Table S4), including bacterial or fungal infections, sinusoidal obstruction syndrome (5% vs 0%, P = .243), thrombotic microangiopathy (9% vs 9%, P = 1.000), and hemorrhagic cystitis (36% vs 39%, P = .786).
3.5 Survival outcomes
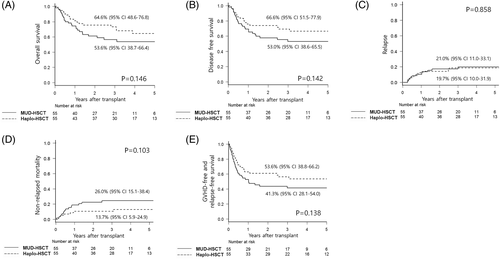
The primary end point of this study was non-inferiority of 3-year OS of Haplo-HSCT compared with MUD-HSCT. At a median follow-up of 47 months (range 12-80 months) for surviving patients, 3-year OS rates for MUD-HSCT and Haplo-HSCT were 57% (95% CI: 42-69) and 73% (95% CI: 59-83), respectively, with no significant difference (P = .146; Figure 1A and Supplementary Table S5). The prespecified boundary to infer non-inferiority was 41%, and the lower confidence limit of Haplo-HSCT did not reach this boundary. We can therefore conclude that Haplo-HSCT is not inferior to MUD-HSCT for this outcome. Five-year OS rates MUD-HSCT and Haplo-HSCT were 54% (95% CI: 39-66) and 65% (95% CI: 49-77), respectively, with no significant difference. Furthermore, adjustment by patients' age and disease status by multivariate analysis even revealed a trend of favorable OS in Haplo-HSCT (Model #1 in Table 2). In terms of secondary end points, DFS (53% vs 67%, P = .142; Figure 1B), CIR (21% vs 20%, P = .853; Figure 1C), and NRM (26% vs 14%, P = .103; Figure 1D) for MUD-HSCT and Haplo-HSCT were not significantly affected by the donor type (Supplementary Table S5). The composite end point of GRFS for MUD-HSCT and Haplo-HSCT was 41% and 54%, respectively, which was also not different between the two groups (P = .138; Figure 1E). However, multivariate Model #1 (Table 2), including all enrolled patients, revealed that DFS and GRFS of Haplo-HSCT were significantly superior to MUD-HSCT. More advanced disease status was a significant factor associated with higher CIR, and translated into inferior OS, DFS, and GRFS, while patients' age was significantly associated with higher NRM.
| Model #1 (n = 110) | n | Overall survival | Disease-free survival | Cumulative incidence of relapse | Cumulative incidence of non-relapse mortality | GVHD-free and relapse-free survival | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Transplant type | |||||||||||
| MUD-HSCT | 55 | 1 | 1 | 1 | 1 | 1 | |||||
| Haplo-HSCT | 55 | 0.53 (0.28-1.0) | .051 | 0.46 (0.24-0.89) | .020 | 0.63 (0.25-1.59) | .326 | 0.42 (0.17-1.02) | .056 | 0.51 (0.28-0.94) | .031 |
| Age | 110 | 1.02 (0.99-1.04) | .161 | 1.02 (1.00-1.05) | .134 | 1.00 (0.97-1.04) | .814 | 1.04 (1.00-1.08) | .049 | 1.01 (0.99–1.04) | .288 |
| Disease state | |||||||||||
| CR1 | 100 | 1 | 1 | 1 | 1 | ||||||
| CR2 | 10 | 2.79 (1.19-6.52) | .018 | 3.30 (1.39-7.84) | .007 | 4.11 (1.27-13.3) | .019 | 2.53 (1.09-5.89) | .031 | ||
| Model #2 (n = 80) | n | Overall survival | Disease-free survival | Cumulative incidence of relapse | Cumulative incidence of non-relapse mortality | GVHD-free and relapse-free survival | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | n | HR (95% CI) | P value | HR (95% CI) | P value | ||
| WT1 levels at pre-HSCT | |||||||||||
| < 250 copies | 69 | 1 | 1 | 1 | 1 | ||||||
| ≥ 250 copies | 11 | 4.20 (1.77-9.97) | .001 | 4.15 (1.76-9.76) | .001 | 7.39 (2.59-21.1) | <.001 | 2.85 (1.23-6.58) | .014 | ||
| Age | 80 | 1.01 (0.97-1.04) | .751 | 1.01 (0.97-1.04) | .770 | 1.00 (0.96-1.05) | .927 | 1.03 (0.99-1.07) | .145 | 1.00 (0.97-1.03) | .993 |
| Conditioning intensity | .028 | .010 | .125 | .039 | |||||||
| MAC (all for MUD-HSCT) | 35 | 1 | 1 | 1 | 1 | ||||||
| RTC (all for Haplo-HSCT) | 38 | 0.45 (0.18-1.11) | .084 | 0.43 (0.28-1.07) | .070 | 0.48 (0.18-1.30) | .147 | 0.52 (0.23-1.22) | .133 | ||
| RIC (all for MUD-HSCT) | 7 | 1.79 (0.55-5.85) | .332 | 2.04 (0.66-6.30) | .215 | 1.51 (0.39-5.84) | .553 | 1.81 (0.60-5.46) | .290 | ||
| Model #3 (n = 80) | n | Overall survival | Disease-free survival | Cumulative incidence of relapse | Cumulative incidence of non-relapse mortality | GVHD-free and relapse-free survival | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | n | HR (95% CI) | P value | HR (95% CI) | P value | ||
| WT1 levels at pre-HSCT | |||||||||||
| < 250 copies | 69 | 1 | 1 | 1 | 1 | ||||||
| ≥ 250 copies | 11 | 4.79 (2.02-11.3) | <.001 | 4.48 (1.93-10.4) | <.001 | 9.82 (3.36-29.6) | <.001 | 3.24 (1.42-7.39) | .005 | ||
| Age | 80 | 1.01 (0.98-1.05) | .369 | 1.02 (0.99-1.05) | .329 | 1.02 (0.97-1.07) | .536 | 1.01 (0.97-1.05) | .524 | 1.01 (0.98-1.04) | .554 |
| Transplant type | |||||||||||
| MUD-HSCT | 42 | 1 | 1 | 1 | 1 | 1 | |||||
| Haplo-HSCT | 38 | 0.33 (0.15-0.76) | .009 | 0.34 (0.15–0.76) | .009 | 0.37 (0.12-1.15) | .085 | 0.30 (0.10-0.97) | .045 | 0.39 (0.18-0.84) | .016 |
- Abbreviations: CI, confidence interval; CR, complete remission; Haplo-HSCT, haploidentical transplant; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; MAC, myeloablative conditioning; MUD-HSCT, matched unrelated transplant; n, number; RIC, reduced-intensity conditioning; RTC, reduced-toxicity conditioning; WT1, Wilms' tumor gene 1.
In non-enrolled patients (n = 109), in whom a patient relapsed before transplant was excluded, 5-year OS rates for MUD-HSCT and Haplo-HSCT were 66% (95% CI: 50-77) and 66% (95% CI: 53-76), respectively, without significant difference, and other survival outcomes were not significantly different (Supplementary Figure S6).
3.6 Role of pre-transplant MRD status by WT1 assay
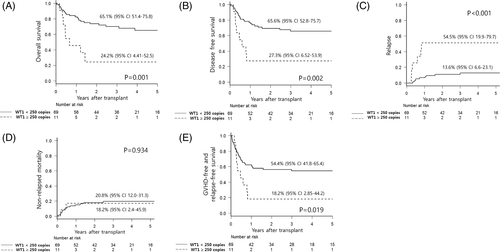
Eighty patients were evaluated with BM WT1 levels before transplant. The cut-off of 250 copies for WT1 levels was suggested by our previous study.11 Pre-transplant WT1 levels ≥250 copies were more frequent in CR2 than CR1 (44% vs 10%, P = .018), whereas there was no difference according to cytogenetic (P = .719) or 2012 NCCN criteria (P = .954). Multivariate models (Models #2 and #3 in Table 2), including those 80 patients, revealed that the pre-transplant WT1 levels ≥250 copies was independently associated with higher CIR, and translated into inferior OS, DFS, and GRFS. Figure 2 shows survival outcomes according to pre-transplant WT1 levels. The role of pre-transplant WT1 MRD assay was also demonstrated in a cohort with non-enrolled patients (Supplementary Figure S7). In addition, adjustment with pre-transplant WT1-MRD status in multivariate Model #3 demonstrated favorable outcomes of Haplo-HSCT compared to MUD-HSCT. However, outcomes of Haplo-HSCT were not significantly different from those of MUD-HSCT in patients with pre-transplant WT1 levels ≥250 copies (Supplementary Figure S8).
4 DISCUSSION
Various platforms of T-cell-replete Haplo-HSCT have been developed worldwide and extended the use of Haplo-HSCT.4, 7 Two major protocols were developed by the University of Beijing using MAC with in vivo T-cell depletion of a PBSC with BM associated with reinforced post-transplant immunosuppression19 and by Johns Hopkins University using a non-myeloablative approach with a high-dose post-transplantation cyclophosphamide (PT-Cy) after infusion of unmanipulated BM,20 which evolved to include MAC and PBSC.21-24 These previous retrospective or prospective studies showed the feasibility of T-cell-replete Haplo-HSCT.4 However, few prospective studies addressed the efficacy in terms of engraftment, anti-leukemia effect, infectious complications, and survival outcomes compared with other donor types. Herein, based on the hypothesis with non-inferiority of 3-year OS in Haplo-HSCT to MUD-HSCT, we prospectively evaluated the transplantation outcomes of Haplo-HSCT and MUD-HSCT for AML in remission with unfavorable prognostic factors, including intermediate and adverse risk groups based on 2012 NCCN criteria and a favorable risk group with CR2. The results proved that Haplo-HSCT, using an RTC with T-cell-replete PBSC, yielded non-inferior OS as a primary end point compared with MUD-HSCT. Indeed, MUD-HSCT has been a preferred choice for AML patients in intermediate and high-risk categories by cytogenetic and/or molecular features in the absence of MSD, based on similar transplant outcomes between the two groups.25-28 However, comparable transplant outcomes between MUD-HSCT and Haplo-HSCT using T-cell-replete grafts in previous retrospective comparisons have disputed the priority of MUD-HSCT over Haplo-HSCT.8, 22, 29-31 The current prospective comparison indicates that our novel Haplo-HSCT protocol using RTC with T-cell-replete PBSC showed comparable graft-vs-leukemia (GVL) effects with MUD-HSCT without concerns of higher toxicity, translating into equivalent OS. These data suggest that patients with AML in remission who require allo-HSCT do not need to search for matched unrelated donors—in particular, patients who urgently need transplantation.
Our Haplo-HSCT protocol is characterized by an RTC regimen, including fractionated TBI of 800 cGy and a relatively lower dose of ATG (5 mg/kg in total) for GVHD prophylaxis, which is unique compared with aforementioned major platforms of T-cell-replete grafts developed by the University of Beijing19 and Johns Hopkins.21-24 The fractionated TBI of 800 cGy was added to a conventional RIC regimen of fludarabine and busulfex to improve the efficacy of eradication of residual leukemic cells without an increase in regimen-related toxicities, and the feasibility was suggested in our previous retrospective studies.8, 9 In the current prospective comparative study, the CIR for the Haplo-HSCT group was comparable to the MUD-HSCT group, who mostly received MAC, without an increase in NRM. Regarding conditioning intensity, our RTC regimen was uniquely positioned between MAC19 and RIC32 by other groups who underwent Haplo-HSCT with T-cell-replete grafts using ATG-based GVHD prophylaxis. The role of TBI 800 cGy for the eradication of residual leukemia was supported by a recent randomized trial showing higher CIR (50%) of conventional RIC regimens without TBI 800 cGy than MAC (17%),33 while another randomized trial showed comparable CIR of a RIC regimen of TBI 800 cGy/fludarabine (28%) with MAC (26%).34 In terms of an ATG dose, we only used 5 mg/kg of ATG for Haplo-HSCT, which was contrasted with other protocols for Haplo-HSCT with T-cell-replete grafts using higher doses of ATG (9 or 10 mg/kg).19, 32 Our data demonstrated that the lower dose of ATG for Haplo-HSCT was adequate to ensure perfect engraftment and similar rates of acute or chronic GVHD with MUD-HSCT, despite the HLA disparity. In the absence of a prospective randomized trial comparing various conditioning intensities for T-cell-replete Haplo-HSCT, the current prospective comparison suggests that RTC is a promising option, incorporating each merit of MAC and RIC in terms of efficacy for the eradication of leukemic cells and safety related with stable engraftment and low rates of GVHD and other transplant-associated complications.
Regarding post-transplant infectious events, no significant differences were found between MUD-HSCT and Haplo-HSCT, with an exception of EBV or CMV infection, which occurred more frequently in Haplo-HSCT. In patients who experienced EBV DNAemia, no patients progressed to post-transplant lymphoproliferative disease, which might be related to the relatively lower dose of ATG. However, CMV infection more frequently required preemptive therapy in Haplo-HSCT, probably due to the higher ATG doses used for Haplo-HSCT (5.0 mg/kg) than MUD-HSCT (2.5 mg/kg). ATG is a polyclonal antibody, interacting with T-cells mainly but also B-cells or dendritic cells,35 which is one of reasons to contribute to the delayed immune reconstitution especially in the Haplo-HSCT. Among immune subsets, we found that early T-cell reconstitution was significantly delayed in Haplo-HSCT compared to MUD-HSCT. CMV reactivation was initiated at a median of 27 days after allo-HSCT, when patients with CMV infection had lower frequencies of T-cells than those without CMV infection. Recent data obtained from another group using T-cell-replete PBSC for Haplo-HSCT showed similar frequency of CMV infection, with MUD-HSCT using identical RIC regimen and ATG dose32; this suggests the importance of ATG doses for CMV infection, rather than HLA disparity or donor types. These data strongly suggest the necessity of more effective preventative and/or preemptive strategies for CMV infection in the Haplo-HSCT group. Given the high risk of CMV infection based on seropositivity for CMV in almost all Koreans or other Asian populations,32, 36, 37 novel approaches for CMV infection, including drug prophylaxis (maribavir or letermovir) or immunotherapy (adoptive T-cell therapy)38 may improve outcomes of Haplo-HSCT in the future.
In addition to rapid availability of Haplo-HSCT compared with MUD-HSCT, an opportunity for discovering innovative forms of immunotherapy by the adoptive cellular therapy of regulatory and conventional T cells could be given in the setting of Haplo-HSCT.7 Haplo-HSCT provides platforms for prophylactic or preemptive interventions for patients at a higher risk of relapse by donor lymphocyte infusion39 or more selected immune cells, such as donor-derived NK cells40 or antigen-specific T-cells,41 which will be facilitated by proper selection of candidates using validated MRD assessment, including PCR-based techniques or multiparameter flow cytometry.10 Our previous study with a large retrospective cohort showed the usefulness of WT1 MRD assay in predicting post-transplant relapse.11 The current prospective study validated the role of pre-transplant WT1 levels as an MRD marker to predict post-transplant relapse and survival outcomes. Given the feasibility of WT1-specific cytotoxic T-cells after allo-HSCT shown by our group,42 prospective trials are needed to investigate whether the risk of relapse in pre-transplant WT1 MRD-positive patients after Haplo-HSCT may be ameliorated by prophylactic post-transplant immunotherapy.
The current study was limited by the lack of randomization of patients to each group. However, both groups were well balanced in prognostic factors related to biological features of AML, such as presenting WBC counts, AML type, cytogenetic or molecular features, and pre-transplant disease or MRD status. A major difference between groups was that more elderly patients were enrolled in Haplo-HSCT than MUD-HSCT, which is even a potential drawback directly related to more post-transplant complications. Indeed, adjustment of patients' age and disease status by multivariate analysis demonstrated favorable DFS and GRFS in Haplo-HSCT. Different conditioning regimens in the MUD-HSCT group need to be considered for the interpretation of data despite mostly MAC regimens, contrasting to a unique RTC in the Haplo-HSCT group. Thus, the difference in outcomes between Haplo-HSCT and MUD-HSCT in this study should be interpreted as results not from different donor type but from each transplant strategy to utilize stem cells from different donors. Adjustment of conditioning regimens as well as patients' age by multivariate analyses supported the significant predictive role of pre-transplant WT1 assay for post-transplant relapse. Another limitation was a potential selection bias caused by the substantial number of patients who met the eligible criteria but refused to participate in this study. The proportion was somewhat higher in the Haplo-HSCT group than MUD-HSCT group. However, separate retrospective analysis for those patients also revealed the equivalence of survival outcomes between MUD-HSCT and Haplo-HSCT, which strongly supported data of the current prospective study.
Several issues need to be further addressed via prospective comparison of Haplo-HSCT using T-cell-replete grafts, such as highly optimized conditioning regimens, novel strategies of CMV prevention, or better GVHD prophylaxis. Indeed, PT-Cy-based GVHD prophylaxis has emerged as a safe and effective alternative due to the limited toxicity of cyclophosphamide for hematopoietic stem cells and functional impairment of alloreactive T-cells and preferential recovery of regulatory T-cells.43 This platform was rapidly adopted by Western countries for successful prevention of severe GVHD.2, 3 A recent data registry, which compares ATG with PT-Cy-based GVHD prophylaxis for AML patients in remission who received Haplo-HSCT with T-cell-replete graft, suggests the superiority of PT-Cy in NRM and severe acute GVHD.44 PT-Cy-based GVHD prophylaxis may be used for our RTC protocol for Haplo-HSCT, which requires prospective comparison with current ATG-based protocol.
This prospective study has demonstrated the equivalence of Haplo-HSCT using a unique RTC regimen with T-cell-replete PBSC compared with MUD-HSCT for AML in remission and validated pre-transplant WT1 quantification as an MRD marker. The advantages of our Haplo-HSCT regimen, such as perfect engraftment with no increased risk of GVHD or securement of GVL effects, combined with rapid availability or the possible use as a platform of immunotherapy, make Haplo-HSCT more attractive than other alternative donors. The application of novel strategies for CMV infection will further improve the outcomes of current protocols for Haplo-HSCT. Prospective studies comparing of ATG- or PT-Cy-based GVHD prophylaxis in T-cell-replete Haplo-HSCT are warranted.
ACKNOWLEDGMENTS
The study design and statistical analyses performed in this article were advised by Catholic Medical Center Clinical Research Coordinating Center.
CONFLICT OF INTERESTS
The authors declare that they have no personal or financial conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




