Hydroxycarbamide decreases the free alpha-hemoglobin pool in red blood cells of adult patients with sickle cell anemia
Sickle cell anemia (SCA) is the most common autosomal recessive red blood cell (RBC) disease in the world characterized by chronic hemolytic anemia, vaso-occlusion crises, acute chest syndrome and chronic visceral complications.1 Sickle cells have a reduced deformability and intravascular lysis which both contribute to the impaired flow of these cells in the microcirculation.2 The SCA is due to the presence of abnormal hemoglobin, HbS (α2βS2, mutation in the HBB gene) in the homozygous state (SS patients) leading to the polymerization of HbS at low oxygen concentration. The frequency and severity of painful episodes both vary widely across patients and over the lifetime in each patient. Note, HbS polymerization is exponentially linked to the HbS concentration in RBCs. Genetic and genome-wide association studies have shown that high levels of fetal Hb (HbF:α2γ2) are associated, on average, with milder SCA phenotypes.3 Also, HbF has a major protective role in lowering SCA severity since it reduces sickling by inhibiting the polymerization of HbS and reducing mean corpuscular HbS concentration.4
The SCA patients can greatly benefit from hydroxycarbamide (HC) therapy. Hydroxycarbamide has multiple cellular effects that lead to a reduction in the frequency and severity of painful attacks. One of the beneficial actions of HC is to increase the synthesis of the γ-globin chain.5 Hydroxycarbamide also reduces the expression of cell adhesion molecules that contribute to vaso-occlusion and the more deleterious dense RBC subpopulation.6 To date, there is no unique biological marker to monitor the response as well as the patients’ compliance to HC treatment. The increased percentage of HbF (%HbF) is a useful marker, but it takes months before its stabilized expression and it cannot reflect short term variations of HC therapeutic effects.
We recently reported a significant increase of the free α-Hb pool values in SCA patients in comparison with the observed values in the reference group that do not exhibit Hb genetic abnormality.7
Owing to the reactivation of the synthesis of the γ-globin chain by HC therapy, we hypothesized that part of these γ-globin chain could associate with free α-chains and thus decrease free α-Hb pool concentration. In a study of children with sickle cell disease, HbF levels varied from 16.2% to 27.8% after 1 year in patients with hydroxyurea treatment.8 We therefore designed an open label trial comparing SCA patients in a stable clinical condition with and without HC therapy, anticipating a 10% to 20% decrease in the α-Hb pool value between the two groups.
This study included 13 SCA adult patients without HC treatment and 10 SCA adult patients with stabilized HC treatment for at least 1 year, followed at the French Sickle Cell Referral Center in Mondor Hospital at Creteil (France). The SCA patients were eligible for the study if they had no α-thalassemia and were in clinical steady-state. Steady state was defined as a visit >8 to 10 weeks after and 2 weeks before a vaso-occlusive crisis or any other clinical event that resulted in hospitalization. Patients were excluded if they had blood transfusion within the previous 3 months, had active chronic viral disease (hepatitis B, C, human immunodeficiency virus), any recent infections or known inflammatory pathologies, hyper or hypothyroidism, any oncologic problem in the last 5 years, and systemic corticosteroid administration. An informed consent was obtained from all subjects in accordance with the Declaration of Helsinki, after approval of the project by the Institutional Review Board. We also studied 10 healthy adult AA recruited as controls at the French Blood Establishment (EFS). All subjects underwent blood sampling on EDTA as anti-coagulant for hematological and biological analysis. For all SCA patients (SS patients), Hb phenotypes were controlled by cation exchange chromatography on an automated analyzer (BioRad Variant II hemoglobin analyzer1 with the dual-kit elution system, Bio-Rad Life Science, Hercules, CA USA). Red blood cell density distributions were assessed in order to determine the percent of dense dehydrated RBCs (%DRBCs) defined as having a density >1.110 g/mL.9
In vitro α-Hb pool measurement assay was evaluated in the RBC lysates of all subjects (SS and AA patients) by the capture of α-Hb subunits onto recombinant α-hemoglobin stabilizing protein (AHSP), the α chaperone beforehand bound to an affinity chromatography support as previously described in detail.10 The values of α-Hb pool were expressed as ppm, equivalent to ng free α-Hb subunits per mg total (α + non-α) Hb subunits per mL of lysed RBCs.
Quantitative variables were expressed as arithmetic mean ± SD. Mann-Whitney U nonparametric tests (n < 30) were used for comparing the SS patient group treated by HC and the SS patient group without HC treatment, or for comparing the SS patient group without HC and the AA healthy subject group. Potential relationships between α-Hb pool values and biologic parameters observed in the two SS patient groups were analyzed by Spearman's rho and Pearson correlation coefficients. In order to evaluate parameters that influence the α-Hb pool levels, we used linear regression analysis. The interaction of all variables with the α-Hb pool values was appraised using a stepwise multivariate analysis, if there was significant correlation between independent variables, only one was entered into the model to avoid multicollinearity. Two-tailed P values <.05 were considered significant. All data processing and statistical analyses were done with Prism 6.00 software (GraphPad Inc, La Jolla CA, USA) and Stata/Se version 12.0 software (StataCorp LP, College Station, TX, USA).
Hematological and molecular data of 13 SS patients without HC treatment and 10 SS patients with HC are summarized in Table S1. In the SS patient group untreated by HC, the RBC counts, average Hb, hematocrit were decreased whereas WBC counts, reticulocytes and platelets were increased compared with the control group as expected for a representative sample of the SCA patient population. As we previously reported,7 a highly significant elevation of α-Hb pool values was observed in the RBCs of the untreated SS patient group in comparison to that observed in the RBCs of AA controls (232 ± 51 ppm vs 88 ± 17 ppm, P < .001).
The comparison of the hematological and clinical data in the two SS patient groups showed that the average Hb, HbF%, MCH and MCV were increased whereas WBC counts and reticulocytes were decreased when the SS patients were treated by HC therapy (Table S1). Notably, the total HbF is increased from baseline in the treated SS group (P< .001). These results are consistent with those reported in the literature.1, 2 In addition the percent of DRBCs was significantly lower in SS patients with HC treatment as previously shown.9 No difference was observed for the ferritin level between the two SS groups.
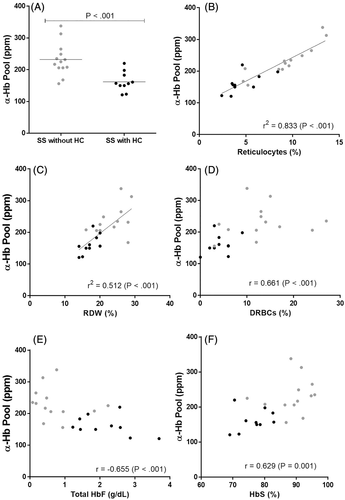
A highly significant decrease of α-Hb pool values was observed in the RBCs of SS patients treated by HC in comparison to that observed in RBCs of untreated SS patients (162 ± 31 ppm vs 232 ± 51 ppm, P<.001) (Figure 1A). For SS patients treated by HC, the α-Hb pool values ranged from 121 to 220 ppm while for untreated SS patients the values ranged from 156 to 338 ppm. A dispersion of α-Hb values was observed for SS patients treated by HC (interquartile range = 43.50), a little more pronounced in untreated SS patients (interquartile range = 51), while this dispersion is smaller in the AA subjects (interquartile range = 22.25).
 Patients with HC treatment (n = 10);
Patients with HC treatment (n = 10);  Patients without HC treatment (n = 13). The value of α-Hb pool is expressed in ppm equivalent to ng α-Hb per mg of total Hb subunits contained per mL of hemolysate in order to take account of the total concentration of Hb varying from one patient to another. The mean for two groups is indicated by the horizontal line. Each point is the mean of two different experiments. The percentage of DRBCs is defined as having a density >1.110 g/mL from the RBC density distributions
Patients without HC treatment (n = 13). The value of α-Hb pool is expressed in ppm equivalent to ng α-Hb per mg of total Hb subunits contained per mL of hemolysate in order to take account of the total concentration of Hb varying from one patient to another. The mean for two groups is indicated by the horizontal line. Each point is the mean of two different experiments. The percentage of DRBCs is defined as having a density >1.110 g/mL from the RBC density distributionsRelationship between the α-Hb pool and 21 clinical and hematological characteristics were analyzed in the two SS patient groups by Spearman's rho and Pearson correlation coefficients (Table S2). One observes trends some more marked than others. Thus, some cellular parameters are associated with α-Hb pool. There was a positive and highly association between α-Hb pool and percent of reticulocytes in SS patients without HC therapy (r = 0.927 P < .001) as suggested in our previous study,7 while for the SS patients with HC therapy, the association is at the limit of significance (r = 0.649, P = .046). There is a trend of association between the red cell distribution width (RDW) with α-Hb pool in SS patients without HC therapy (r = 0.522, P = .07); but this association is more pronounced in treated SS patients (r = 0.745, P = .017). No significant association was observed between the α-Hb pool and percentage of DRBCs, a subpopulation of RBCs which is an important parameter intricately involved in SCD chronic organ complications.9 In the first study of SS patients in clinical steady-state,7 significant association was observed between the α-Hb pool and %DRBCs but SS patients with three α-globin genes and four α-globin genes were combined in one single group (n = 21).
Due to the small number of the patients in each group, correlations between the α-Hb pool and different data were re-analyzed considering treated and untreated patients in one single group (n = 23) (Table S2). Thus, the α-Hb pool is highly correlated with the percent of reticulocytes (r2 = 0.833, P < .001) (Figure 1B) and with RDW (r2 = 0.512, P < .001) (Figure 1C). We also found an association between α-Hb and %DRBCs (r = 0.661, P < .001) (Figure 1D) or total HbF (r = −0.655, P < .001) (Figure 1E) or % HbS (r = 0.629, P = .001) (Figure 1F). Cellular parameters (WBC, % reticulocytes, % RDW and % DRBCs) and % HbA2 and % HbS were positively correlated with α-Hb pool, whereas MCH and HbF (expressed in percent, or total value, or mean corpuscular per cell) were negatively correlated. Variables significantly correlated with α-Hb pool (P < .007) according to our univariate analysis are reported in the Table S3. Multivariate analysis shows significant associations between α-Hb pool values and % reticulocytes, RDW and % HbF (Table S3). Concerning the correlation between HbF and the α-Hb pool, it is well known that the formation of the tetramer α2βS2 is less efficient than the normal tetramer α2βA2, the βS subunits having a lower affinity than the normal chain βA. It is also known that the fetal γ genes are reactivated by HC therapy. These γ-chains also exhibiting a high affinity for α subunits could associate with a part of excess of α-Hb to form the α2γ2 HbF tetramer explaining the decrease in the α-Hb pool observed in treated SCA patients.
The α-Hb pool could be a resultant of various genetic factors (HbF, α-thalassemia, AHSP, HS-40, and others) modulating the SCA phenotype. The presence of multiple modifiers and their interactions makes interpretation more difficult. However, in sickle cell disease, some cytosolic RBC proteins are upregulated such AHSP,11 a private chaperone for α-globin. In 2015, Mahmoud et al. compare AHSP level between patients with beta-thalassemia and SCA.12 They found that AHSP gene expression was higher in patients with SCA vs thalassemia. It was also higher in non-transfusion dependent beta-thalassemia patients and on hydroxyurea therapy.12 This factor could be a secondary compensatory mechanism in RBCs to counterbalance the excess α-globin chains in these patients. That remains to be explored. However, these interesting results must be interpreted with care given the small number of patients, and it will be important to study a larger SS patient population.
In summary, this study demonstrates for the first time that the SS patients treated by HC have a decreased soluble α-Hb pool compared to that observed in untreated SS patients. Thus, this soluble α-Hb pool could be a surrogate marker to monitor the HC response, which is known to be variable and inconsistent from one patient to another, as well as to follow the patients’ compliance to HC treatment. Subsequently a longitudinal study after introduction of HC treatment will allow study of the evolution of soluble α-Hb pool in response to therapy.
ACKNOWEDGEMENTS
This work was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm), France, the Etablissement Français du sang (EFS), the University of Paris-Est Créteil (UPEC). We thank Dr. Serge Pissard for the α-globin locus studies. We also thank the patients, nurses, and physicians at the French Sickle Cell Referral Center who contributed to the management of patient care.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Elisa Domingues-Hamdi collected the data of AA controls and carried out all lysates of RBCs; Elisa Domingues-Hamdi and Corinne Vasseur performed the measurement of the α-Hb pool; Sadaf Pakdaman collected the data of SS patients; Stéphane Moutereau performed RBC density distributions. Anoosha Habibi, Pablo Bartolucci and Frédéric Galactéros were in charge of the selection of patients; Véronique Baudin-Creuza designed the study and collected the data. Elisa Domingues-Hamdi, Corinne Vasseur, Véronique Baudin-Creuza analyzed the data. Véronique Baudin-Creuza, Corinne Vasseur and Frédéric Galactéros wrote the paper; All authors critically revised the manuscript and approved the final version of the manuscript.




