Phase 1 study of combinatorial sorafenib, G-CSF, and plerixafor treatment in relapsed/refractory, FLT3-ITD-mutated acute myelogenous leukemia patients
Funding information: Foundation for the National Institutes of Health, Grant/Award Numbers: CA016672, CA055164, R01FD003733, R21CA143805
Abstract
Stroma-leukemia interactions mediated by CXCR4, CD44, VLA4, and their respective ligands contribute to therapy resistance in FLT3-ITD-mutated acute myelogenous leukemia (AML). We conducted a phase 1 study with the combination of sorafenib (a FLT3-ITD inhibitor), plerixafor (a SDF-1/CXCR4 inhibitor), and G-CSF (that cleaves SDF-1, CD44, and VLA4). Twenty-eight patients with relapsed/refractory FLT3-ITD-mutated AML were enrolled from December 2010 to December 2013 at three dose levels of sorafenib (400, 600, and 800 mg twice daily) and G-CSF and plerixafor were administered every other day for seven doses starting on day one. Sorafenib 800 mg twice daily was selected for the expansion phase. While no dose-limiting toxicities (DLT) were encountered in the four-week DLT window, hand-foot syndrome and rash were seen beyond the DLT window, which required dose reductions in most patients. The response rate was 36% (complete response (CR) = 4, complete remission with incomplete platelet recovery (CRp) = 4, complete remission with incomplete hematologic recovery (CRi) = 1, and partial response (PR) = 1) for the intention to treat population. Treatment resulted in 58.4 and 47 mean fold mobilization of blasts and CD34 /38- stem/progenitor cells, respectively, to the circulation. Expression of the adhesion molecules CXCR4, CD44, and VLA4 on circulating leukemia cells correlated negatively with the mobilization of CD34+/38-, CD34+/38−/123+ “progenitor” cells (all P ≤ .002). Mass cytometry analysis of sequential samples from two patients demonstrated resistance emerging early on from sub-clones with persistent Akt and/or ERK signaling. In conclusion, the strategy of combined inhibition of FLT3 kinase and stromal adhesive interactions has promising activity in relapsed/refractory, FLT3-ITD-mutated AML, which warrants further evaluation in the front-line setting.
1 INTRODUCTION
The chemokine ligand SDF-1 and its receptor CXCR4 are highly relevant in FLT3-ITD-mutated acute myelogenous leukemia (AML).1, 2 Among AMLs, FLT3-ITD-mutant AML has the highest expression of CXCR4, and in a multi-variate analysis high CXCR4 expression superseded the adverse prognostic impact of FLT3-ITD mutation.2 Activating FLT3 mutations induce proliferation while SDF-1/CXCR4 signaling is pivotal in AML cell homing and migration.3 FLT3 ligand (FL) inhibits apoptosis by influencing Bcl-2 and BAX levels in AML cells.4 Co-activation by FL and SDF-1 increases pro-survival signals, including phosphorylation of MAPK and Akt,5 which are signals that contribute to stroma-mediated resistance to AML therapy. The pronounced hypoxia in bone marrow (BM) further contributes to increased expression of CXCR4 in AML cells and also enhances chemoresistance.6 Thus, disruption of the SDF-1/CXCR4 axis can overcome stroma-mediated chemoresistance by mobilizing AML cells from the protective sanctuary of the BM microenvironment.7 The SDF1/CXCR4 axis also increases critical anti-apoptotic signaling in AML cells via the SDF1-CXCR4-Yin/Yang1-let7a-cMyc/Bcl-XL axis, as reported by us previously.8 We and others have reported that CXCR4 inhibition alone with the small molecule LY2510924 or the peptidic inhibitor BL-8040 has anti-leukemic activity in vivo,9, 10 and that BL-8040 or plerixafor (a small molecule inhibitor) can enhance the clinical activity of chemotherapy.11, 12 CXCR4 inhibition downregulates ERK, Bcl-2, Mcl-1, and cyclin D1 via altered miR15a/16 expression.10 Finally, pro-survival signals through CXCR4, VLA4, and CD44 can also be countered by elastase-mediated cleavage affected by granulocyte colony stimulating factor (G-CSF).13, 14
Stromal protection is particularly relevant in FLT3-ITD-mutated AML, and we and others have shown that disruption of stromal protection by CXCR4 inhibition enhances response to FLT3 inhibitor treatment in pre-clinical AML models.7, 13-15 Plerixafor, a small molecule inhibitor of SDF-1 binding to CXCR4, is approved in combination with G-CSF for autologous stem cell mobilization prior to transplantation in patients with non-Hodgkin's lymphoma and multiple myeloma. While proteolytic cleavage of adhesion molecules contributes to mobilization by G-CSF,13, 14 G-CSF-mediated reduction in SDF-1 levels in the BM further facilitates this process.
The leukemogenic role of FLT3-ITD mutations is evident by remissions induced by FLT3-ITD inhibitors.16, 17 Sorafenib was first reported by us as a highly effective inhibitor of ITD-mutated FLT318 and, as a single agent in relapsed/refractory FLT3-ITD-mutated AML, can induce remissions in about 10% of patients.16 Combinations of sorafenib with chemotherapies or hypomethylating agents in front-line or salvage setting yield higher rates of responses than sorafenib alone.19, 20 Our preclinical data indicate that concomitant CXCR4 inhibition with plerixafor combined with G-CSF can overcome stroma-mediated resistance to sorafenib in FLT3-mutated AML, an effect that extends to hypoxic conditions, and translates into improved survival of FLT3-ITD-mutant AML bearing mice.7 Therefore, we hypothesized that the combination of sorafenib with plerixafor and G-CSF will increase remission rates in relapsed/refractory FLT3-ITD-mutated AML compared to those achieved with sorafenib alone, and that this can serve as a backbone for the development of combinatorial strategies21, 22 with other chemotherapeutics or targeted agents as a frontline approach in the therapy of FLT3-mutated AML.
2 METHODS
2.1 Patients
Patients with relapsed or refractory AML with FLT3-ITD mutation (with or without TKD mutation), age ≥ 18 years were eligible. Details of eligibilities are in the Supplemental Methods. All patients were registered in an Institutional Review Board-approved protocol and signed their informed consent. The trial was registered at clinical trials.gov (NCT00943943). The trial is closed to new patient entry.
2.2 Treatment plan
In cycle 1, the first dose of sorafenib was 12 hours after G-CSF and plerixafor injections to allow study of leukemia cell mobilization. The tested dose levels of oral sorafenib were 400 mg, 600 mg, and 800 mg administered twice-daily, continuously. The G-CSF dose was 10 mcg/kg SQ, and the plerixafor dose was 240 mcg/kg SQ, both administered every other day for seven doses starting on day 1. Cycle length was 28 days and treatment was continued for responders except the ones proceeding to transplant. Dose reductions and supportive care are detailed in the Supplemental Methods.
2.3 Flow cytometry
Flow cytometry studies were conducted on peripheral blood (PB) (at 0, 5, and 12 hours post G-CSF/plerixafor administration on days 1, 2, 4, and 14) and bone marrow (BM) (baseline and day 14-17) samples as described in the Supplemental Methods.
2.4 Fluorescent in situ hybridization (FISH)
For patients with a cytogenetic abnormality for which a probe was available, FISH studies were carried out as described previously23 (and Supplemental Methods) to confirm preferential mobilization of leukemic cells.
2.5 FLT3 mutant/wild-type allelic ratio
FLT3-ITD, codon 835/838 TKD mutations, and allelic ratio were determined from genomic DNA extracted from BM aspirate as described previously.24
2.6 CyTOF assay and data analysis
Cell preparation for CyTOF assay was completed as described previously25 (and in Supplemental Methods). The antibodies used in the CyTOF analysis are presented in Table S1. CyTOF data were normalized by Matlab-based software Bead-normalization, and processed by FlowJo v10 (BD Biosciences-SG, Singapore). Data were exported to SPADE26 to build a 100-node tree using surface markers clustered by the K-means clustering algorithm and branched using a minimal spanning tree algorithm. The subsets identified by SPADE were confirmed by viSNE27 and further analyzed, plotted by GraphPad Prism 6 (GraphPad Software, San Diego, CA).
2.7 Response evaluation
Evaluation of response was done according to the modified International Working Group (IWG) criteria28 (see Supplemental Methods).
2.8 Statistical design and analysis
This was a phase 1 trial with traditional “3 + 3” design (see Supplemental Methods). Pearson correlations were used to evaluate the relationship of baseline expression of surface markers. The associations between baseline values and fold changes in PB of leukemic cells were tested by linear regression. Wilcoxon rank-sum tests were used to assess the association between these measurements and overall response. Logistic regression analyses were used to explore the association between measurements and overall response. Statistical analyses were performed using SAS 9.3 (SAS Software, Cary, NC), and graphics were created using Microsoft Excel. Besides P-vales, multiple testing was adjusted by also reporting q-values (computed by R package q-value).
3 RESULTS
Between December 2010 and December 28, 2013 patients were treated. The patient characteristics are summarized in Table 1. The median patient age was 58 years (range 18 to 84 years), 16 (57%) were females, and the median number of prior therapies was two (range one to six). Eleven (39%) patients had prior FLT3 inhibitor therapy, including two patients who received more than one such inhibitor. Ten (36%) patients had prior allogeneic stem cell transplant (SCT). The median FLT3-ITD allelic ratio was 0.435 (range 0.007-0.889), four (14%) patients had an additional FLT3 D835 mutation, and concomitant NPM1 mutation was present in nine patients (32%).
| Parameters | Number (%) | Number (%) |
|---|---|---|
| Age years, median (range) | 58 (18-85) | |
| Gender | ||
| Male | 12 (43) | |
| Female | 16 (57) | |
| WBC (x109/L) (median, range) | 5.5 (0.2-36.8) | |
| Prior therapy, median (range) | 2 (1–6) | |
| ECOG performance status | ||
| 0–1 | 46 (92) | |
| 2 | 4 (8) | |
| Cytogenetics | ||
| Diploid | 15 (54) | |
| Ch6 | 4 (14) | |
| Ch3 | 6 (13) | |
| FLT3 Mutation | ||
| ITD only | 24 (86) | |
| ITD + D835 | 4 (14) | |
| ITD allelic ratio (median, range) | 0.44 (0.007–0.89) | |
| NPM1 mutation | 10 (36) | |
| Prior FLT3 inhibitor | 11 (39) | |
| Prior SCT | 10 (36) |
3.1 Dose escalations
Three patients were enrolled at each dose level of sorafenib at 400 mg and 600 mg twice daily (BID). Three additional patients at 600 mg dose level were not evaluable for dose limiting toxicities (DLT) since their disease progressed before completing cycle 1. Because of the lack of DLT in cycle 1, a sorafenib dose of 800 mg twice daily was chosen for expansion. A total of 19 patients were accrued at the sorafenib dose level of 800 mg twice daily, which was then considered the maximum tolerated dose.
3.2 Dose reductions/interruptions
Sorafenib dose reductions were necessary in 10 patients; 9/10 at the 800 mg BID dose level. Reasons for the reduction included skin toxicities (hand-foot syndrome and/or rash, six patients), grade 3 hyperbilirubinemia (two patients, one with graft-vs host disease exacerbation), grade 2 peripheral neuropathy (one patient), and hypertension (one patient). Among these 10 patients, four required reduction in the doses of plerixafor and G-CSF for bone pain (one patient), rash (one patient), high white blood cell count (WBC, one patient), and reduced creatinine clearance (one patient). Additionally, one patient needed a reduction in the plerixafor and G-CSF dose, without modification of the sorafenib dose, due to leukocytosis.
3.3 Toxicities
The toxicities considered to be related to the study drugs are summarized in Table S2. While no DLT defining events were encountered in cycle 1, allowing escalation of sorafenib per design to the 800 mg level, grade ≥ 3 non-hematological toxicities beyond cycle 1 were encountered in 20 patients. Grade 3 skin toxicities (hand-foot syndrome, desquamating rash) and fatigue were encountered in three patients followed by grade 3 cardiac arrhythmias in two patients (atrial fibrillation in one patient and supra-ventricular tachycardia in one patient). Two patients developed reversible renal failure, one because of hypotension/hypovolemia and the other in the setting of Pneumocystis pneumonia after receiving high-dose trimethoprim and sulfamethoxazole. Three patients developed transient grade 3 or 4 liver enzyme elevations, and one patient developed pericardial and pleural effusion shortly after completion of study, which required drainage. Three patients experienced grade 3 bone pain that was attributable to plerixafor and GCSF and required administration of oral narcotics for management of the symptoms.
Five patients died while on study. Two deaths were from sepsis, one from Pseudomonas aeruginosa infection, and the other two from Trichosporon fungemia infection. Three other deaths were from progressive disease (two patients developed intra-cranial hemorrhage in the setting of profound thrombocytopenia, and the third was a sudden death from cardio-respiratory arrest that happened at home). One additional patient died shortly after study discontinuation from diffuse alveolar hemorrhage with profound thrombocytopenia. This patient also developed a subdural hematoma without any midline shift. Nineteen patients required hospitalization during the study for neutropenic infectious complications. Two infection-related deaths are reported above. Four patients experienced ≥grade 3 hyperleukocytosis (WBC >100 X 109/L) requiring short-term use of hydroxyurea for count control, and one of them continued treatment with sorafenib alone. Two of the other three required reduction in plerixafor/GCSF and/or sorafenib dose reduction.
3.4 Responses
Survival of all patients from the start of study therapy is shown in Figure 1. None of the three evaluable patients with FLT3 D835 mutation responded. Among 28 intension-to-treat patients, the response rate was 37% (CR = 4, CRp = 4, Cri = 1, PR = 1). The median number of cycles to response was two (range 1 to 10), and the median number of cycles of therapy received by responders was five (range 3 to 23). Four patients were considered not evaluable for a response: three because of early discontinuation not related to disease progression (one patient withdrew consent, one patient developed seizure and alveolar hemorrhage, and one patient experienced endocarditis with stroke) and one because of early death from sudden respiratory arrest. Four responders had prior allogeneic SCT. Among patients not previously exposed to FLT3 inhibitors, seven of 14 (50%) responded (CR = 4, CRp = 1, morphologic leukemia free state (MLFS) = 1, PR = 1) and three of 10 (33%) who received prior FLT3 inhibitor therapy (two patients received midostaurin and one sorafenib) also responded. The median duration of response was 5.3 months (range 0.5 to 16 months).
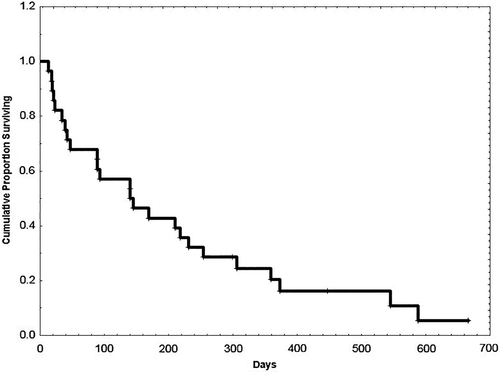
All except one patient have left the study. One patient with CR continued beyond 56 months of therapy and one patient died in CR from SCT-related complications. Three patients had acquired D835 mutation at relapse. All but two responders remained FLT3-ITD positive during therapy. Two patients with pre-treatment FLT3-ITD allelic burdens of 0.634 and 0.083 became FLT3-ITD negative on treatment. The second of these two patients continues in remission for 56 months (as above). The other relapsed with re-emergence of FLT3-ITD positivity and acquisition of D835 mutation at 16 months.
3.5 Cell mobilization
Mobilization of leukemia cells was defined as the maximum ratio to its baseline value. Mobilization to peripheral circulation between day 1 and day 14 was clearly evidenced by a several-fold change in absolute blast count (ABC; mean 58.4, median 42.43, range 9.25 to 207.07), CD34+ cells (mean 98.6, median 4.23, range 0.45 to 1731.49), CD34+/38- cells (mean 47, median 6.8, range 0 to 421.27), CD34+/38-/CD123+ cells (mean 77.5, median 11.09, range 1.11 to 883.52), CD44+ cells (mean 37.4, median 6.97, range 0.44 to 613.84), VLA4+ cells (mean 31.2, median 4.97, range 0.47 to 488.12), and CXCR4+ cells (mean 70.6, median 24.2, range 0.75 to 636.65) (Table 2). Figure S1A,B depict the fold changes in these parameters in PB from baseline, and 5 and 12 hours post G-CSF/plerixafor treatment (prior to sorafenib) on day 1, and 12 hours post G-CSF/plerixafor administration on day 2.
| Max. Fold Change Over Baseline | Mean (Min, Max) | Median |
|---|---|---|
| At day 1, 12 hours | ||
| CD34+ | 23.6 (0.31, 349.77) | 1.5 |
| CD44+ | 10.6 (0.32, 157.55) | 1.4 |
| VLA4+ | 6.7 (0.32, 101.25) | 1.2 |
| CXCR4+ | 43.6 (0.57, 636.65) | 3.9 |
| CD34+/38- | 24.2 (0, 421.27) | 1.6 |
| CD34+/38−/123+ | 50.96 (0.18, 883.52) | 1.92 |
| Within Cycle 1, day 1–2 | ||
| WBC | 8.84 (0.42, 73) | 3.38 |
| ANC | 7 (0.04, 33.32) | 3.87 |
| AMC | 8.8 (0, 80.3) | 3.54 |
| ABC | 34.3 (2.23, 109.91) | 18.25 |
| CD34+ | 72 (0.4, 1329.84) | 3.21 |
| CD44+ | 23 (0.44, 379.98) | 3.91 |
| VLA4+ | 16.8 (0.41, 227.5) | 3.62 |
| CXCR4+ | 55 (0.75, 636.65) | 7.86 |
| CD34+/38- | 31 (0, 421.27) | 4.46 |
| CD34+/38−/123+ | 58 (0.37, 883.52) | 7.25 |
| Within Cycle 1, day 1-14 | ||
| WBC | 19.9 (0.42, 135) | 4.79 |
| ANC | 38.9 (0.04, 622.18) | 6.46 |
| AMC | 67 (0.99, 172.2) | 5.03 |
| ABC | 58.4 (9.25, 207.07) | 42.43 |
| CD34+ | 98.6 (0.45, 1731.49) | 4.23 |
| CD44+ | 37.4 (0.44, 613.84) | 6.97 |
| VLA4+ | 31.2 (0.47, 488.12) | 4.97 |
| CXCR4+ | 70.6 (0.75, 636.65) | 24.2 |
| CD34+/38- | 47 (0, 421.27) | 6.8 |
| CD34+/38−/123+ | 77.5 (1.11, 883.52) | 11.09 |
The maximum fold changes for CD34+, CD44+, VLA4+, and CXCR4+ cells within the first 2 days of treatment were negatively correlated with the log-transformed baseline CD44+, VLA4+, and CXCR4+ cell numbers in PB (Table S3). Univariate regression analysis presented in Table S4 showed that the baseline CXCR4 level was negatively associated with the fold increase of PB WBC. A percent change in CXCR4-expressing cells was associated with −0.05-fold change in WBC (P < .001). However, none of the mobilization parameters or fold changes could be directly correlated with a response.
3.6 Cell mobilization by FISH
Nine patients had cytogenetic abnormalities detectable by FISH probes. The maximum median fold increase in the absolute number of PB FISH positive blasts by cycle 1, day 14 compared to baseline was 6.1 (range 2.1 to 28; Figure S1C), while FISH-negative cells were mobilized only 3.3-fold (range 0.8 to 14.6), demonstrating the preferential mobilization of leukemic blasts. In seven of nine patients, maximum mobilization by this parameter occured by day 4. The fact that two patients became FLT3-ITD negative suggests that long-term eradication of the FLT3-mutated leukemic clone was possible using this approach of combining an FLT3 inhibitor with microenvironment-targeting agents.
3.7 Mass Cytometry Analysis (CyTOF)
Using CyTOF data combined with SPADE25 and viSNE27 analysis, we characterized subpopulations of AML cells. The dynamics of response and signaling from two patients over the initial course of therapy was examined. This could not be performed on samples from other patients as they received hydroxyurea for induction therapy. In patient 27, SPADE identified three distinct subsets of AML cells in the BM sample at baseline: A = CD34++CD33++, B = CD34−/+CD33+++, and C = CD34-CD33- (confirmed by viSNE) (Figure S2A). Akt (and its downstream targets) and Stat5 phosphorylation was highest in the CD34−/+CD33+++ population (Figure S2B).
The dynamics of quantitative change in these defined subsets were variable between PB and BM (Figure S3A). By day 14 of cycle 1, there was a 99% reduction in both A and B subsets in PB. However, in the BM, while the B subset decreased from 19% (baseline) to 2% (day 14), the A subset was nearly unchanged (28% baseline to 26% at day 14) (Figure S3A); the C subset increased after treatment in both PB and BM samples. The overall reduction of initially-dominant B subset was in agreement with blast count reduction in PB and BM on day 14 (Table S5). pAKT, p4EBP1, pS6, pSYK, and pSTAT3 levels decreased in BM on treatment, while pSTAT5 increased in all three subsets of cells (Figure S3B). pERK was upregulated in the residual cells of the initially-dominant subset B on day 14 BM samples (Figure S3B), as previously reported in sorafenib16 and other FLT3 inhibitor trials.29 SPADE and viSNE identified two resistant subsets, R1 and R2 (Figure 2A). The R1 subset, though suppressed in PB, was remarkably increased in BM at day 14. The R2 subset was miniscule in either the PB or in BM at baseline, but it was over 10% at day 14 (Figure 2B). Intracellular levels of pAKT, pERK, pSYK, and pSTAT5 were higher in R1 and R2 at baseline compared to the CD34++CD33++ cells (Figure 2C).
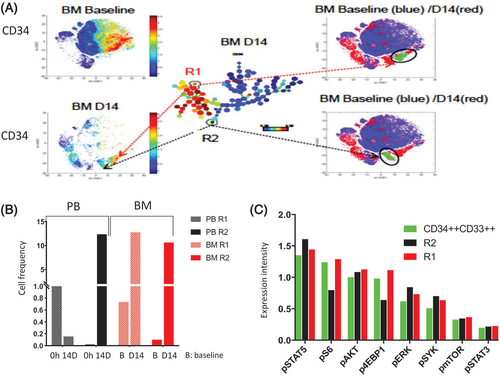
In patient 26, four distinct leukemic subsets were identified (both SPADE and viSNE): (a) CD34+, (b) CD34-CD44+CD11b+, (c) CD34-CD11b+, and (d) CD34-CD8a+ (Figure S4A). Figure S5A-D depicts the quantitative changes in the subpopulations of PB and BM and their signaling patterns while on treatment. Two resistant subsets (R1 and R2) (Figure S6A) were detected in PB and BM at baseline, and remained on day 14 in the subsequent samples, albeit at a similar or lower frequency (Figure S6B,C). Compared to the CD34+ cells, the intensity of intracellular pAKT, pS6, and pERK expression was higher in the R2 subset (Figure S6D).
4 DISCUSSION
Mobilization of BM-resident AML blasts by blocking the SDF-1/CXCR4 axis alone has met with limited success.12 Here, we added G-CSF to the CXCR4 inhibitor plerixafor, since G-CSF, through activation of elastase, can cleave SDF1, CD44, and VLA-4, which are all important components of the AML microenvironment,13, 14 as supported by our preclinical data.7, 8 With a chemotherapy-free regimen combining plerixafor, G-CSF, and sorafenib, 36% of patients with relapsed/refractory FLT3-ITD-mutant AML (including patients with prior FLT3 inhibitor exposure) responded. This response rate is higher than that achieved with sorafenib alone,16, 19 and it promoted a statistically significant survival advantage for the responders. This mobilization strategy expectantly made FLT3-ITD-mutated AML cells more sensitive—not only to sorafenib, but we envision that it would do likewise with chemotherapy. Consequently, our data provide the possibility of combining plerixafor/GCSF/FLT3 inhibition with chemotherapeutic agents or hypomethylating agents20 in the frontline setting.
There was clear mobilization of leukemic blasts and “progenitor” cells (CD34+/38-, CD34+/38−/123+), a process that started as early as 5 hours after G-CSF and plerixafor administration. The strong negative correlation of baseline expression of adhesion molecules like CXCR4, CD44, and VLA4 with mobilization of “progenitor” cells to PB indicates that AMLs with high expression of adhesion molecules are the most difficult to mobilize. Higher mobilization of blasts in this study compared to plerixafor alone supports the addition of G-CSF to CXCR4 inhibitor.12 Mobilization of blasts with AML-related cytogenetic abnormalities in the PB further attests to the ability of this combination to preferentially mobilize leukemic cells from sanctuaries. These mobilization results are encouraging, as G-CSF and plerixafor were only administered every other day: perhaps a sub-optimal schedule given the short receptor occupancy of plerixafor.
Presence of D835 mutations in the tyrosine kinase domain in FLT3 confers resistance to potent FLT3 inhibitors like sorafenib and quizartinib.30 As expected, several of the responders acquired D835 mutations at the time response to therapy was lost. Other patients who failed to respond, or who lost response without D835 mutation, could have had point mutations at (a) site(s) other than D835,31 or other non-FLT3 mutations.32 It is interesting that two patients with prior exposure to FLT3 inhibitors, and lacking D835 mutation, responded. This included one patient with prior exposure to sorafenib, suggesting that a deeper FLT3 inhibition or sensitization strategy could potentially salvage some of the patients who do not acquire resistant mutations.
There was a clear limitation to our attempt to maximize FLT3 inhibition by escalating the sorafenib dose to 800 mg twice daily, primarily because of the skin-associated toxicities encountered beyond the DLT window of cycle 1. In our phase 1 trial with sorafenib as single agent, the maximum tolerated dose was 400 mg twice daily, and that is the dose we utilized in our combinatorial trial with azacitidine and sorafenib.16, 19 There are possibly two mitigations to this scenario: first, to use the 800 mg twice-daily dose of sorafenib in cycle 1 and reduce the dose beyond the first cycle to maximize FLT3 inhibition initially; the second would involve the use of a 400 mg twice-daily dose of sorafenib as this will result in less dosing interruptions and potentially allow consistent FLT3 inhibition.
Unfortunately, the mass cytometry (CyTOF) studies were limited to two patients because the majority of patients received hydroxyurea for initial induction, rendering the samples unusable. From a patient safety perspective, the initial induction therapy was necessary since we were concerned that the mobilization strategy had a potential for leukostasis. Given this limitation, our data highlight the ability of CyTOF analysis to identify complex leukemic subpopulations based on surface markers and allow us to monitor their intracellular signaling and the quantitative changes associated with these signaling processes. CyTOF analysis could also discern small populations, beyond the resolution of conventional flow cytometry, that persist or expand during therapy that potentially contributes to resistance. Biologically, increased pAKT and pERK, and/or pSYK and pSTAT5, in resistant subpopulations during the first cycle of treatment argues strongly for combined pathway targeting from the onset of therapy.
In this trial, there was no correlation between pre-treatment FLT3-ITD allelic burden and response. Barring few exceptions, most responders did not have substantial reduction of FLT3-ITD allelic burden (Table S1), suggesting persistence of residual disease and/or clinical remission through differentiation of leukemic clones. Given this fact, relatively short remissions are not surprising. Similar findings are reported from trials with IDH inhibitors, where response was not always associated with allelic burden reductions.33 Unfortunately, most single-agent FLT3 inhibitor trials do not report changes in allelic burden, which precludes comparison with our results. The addition of chemotherapy or hypomethylating agents with FLT3 inhibitors may enhance reduction of the mutant FLT3 allelic burden.
Finally, it is debatable that sorafenib is the best FLT3-ITD inhibitor available, but the concept of targeting microenvironment in FLT3-mutated AML that we present here is likely generalizable with other FLT3-ITD inhibitors. In conclusion, the combination of sorafenib with GCSF and plerixafor results in mobilization of leukemia cells from BM to peripheral circulation and encouraging rates of response in relapsed, refractory AML with FLT3-ITD mutations. Our data provide a rationale for combined inhibition of FLT3-ITD and stroma-mediated signaling that can potentially be added to chemotherapeutic or epigenetic agents in the frontline setting.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (CA055164, R01FD003733, and R21CA143805) and the MD Anderson Cancer Center Support Grant (CA016672), Cancer Prevention Research Institute of Texas (CPRIT, RP121010), and the Paul and Mary Haas Chair in Genetics (all to MA). The clinical trial was supported by Genzyme Corporation and Bayer US, who also provided plerixafor and sorafenib, respectively. We thank Jared Burks, Duncan Mak from the MD Anderson Cancer Center Flow Cytometry Core for assistance with the CyTOF technology.
DISCLOSURES
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Designed research: GB, MK, and MA; manuscript preparation: GB and MA; manuscript editing: JC, MK, FR, TK, ND, and HK; data analysis: GB and MA; CyTOF analysis: ZZ; recruited patients: JC, MK, FR, TK, ND, and HK; data review: JC, FR, TK, ND, and HK; statistical analyses: H-CC; mutational analysis: XH; research nurse: KPP and MAK; analyzed cell mobilization data: TMQ and R-YW; performed flow cytometry: TMQ; performed FISH analysis: R-YW; conceived study concept: MA; principal investigator: MA.




