Genetic lesions disrupting calreticulin 3′-untranslated region in JAK2 mutation-negative polycythemia vera
Funding information: Associazione Italiana per la Ricerca sul Cancro, Grant/Award Number: AIRC-IG-11949; Fondazione Cassa di Risparmio di Perugia; Sapienza Università di Roma, Grant/Award Numbers: RM11715C81899055, RM11816436B700D1
To the Editor:
Philadelphia-negative myeloproliferative neoplasms (MPNs) originate from transformed hematopoietic stem cells (HSC), retaining the capacity for multilineage differentiation and effective myelopoiesis.1 Myeloproliferative neoplasms include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), characterized by an excessive production of terminally differentiated red blood cells, platelets and replacement of the bone marrow by fibrotic tissue. Despite the diverse phenotypes, MPNs share major genetic, molecular and pathophysiological features, including clonal hematopoiesis, triggered by mutually exclusive somatic driver mutations in the Janus kinase 2 (JAK2), thrombopoietin receptor (MPL) and calreticulin (CALR) genes, all deregulating the JAK2/STAT signaling pathway.1 The CALR mutations are found in more than 60% of ET and 80% of PMF not carrying JAK2 or MPL mutations, but are rare or absent in PV.1-3 They mainly consist of exon-9 deletions and insertions, causing a frameshift that removes the KDEL endoplasmic reticulum-retention motif and generates a common novel positively-charged C-terminal sequence. This constitutively activates the MPL-JAK/STAT signaling pathway, and induces ET-like and PMF-like phenotypes in vivo1 (and references therein). The frameshift caused by various CALR mutations also converts into coding sequence the first 31 nucleotides of the 3′-untranslated region (3′-UTR) mRNA, whose functional role remains unknown. Here, we report the identification and functional characterization of noncanonical CALR mutations disrupting such CALR 3′-UTR region in JAK2-mutation-negative patients presenting an MPN disease resembling PV.
The 3′-UTRs, the noncoding parts of mRNAs, are emerging as major regulators of phenotypic diversity of higher organisms, mainly by controlling mRNA stability, transport, intracellular localization and translational efficiency.4 Their secondary structure regulates the binding of RNA-binding proteins or microRNAs to mRNAs.4, 5 By binding phylogenetically conserved regions on 3′-UTR mRNAs, these molecules regulate fundamental biological processes, including hematopoietic lineages development and differentiation; their disruption has been implicated in oncogenesis.4, 5
We genotyped driver mutations in blood cells from 286 consecutive MPN patients, diagnosed at our institution following 2016 WHO recommendations as described in Appendix S1. Patients' cohort included 64 PV, 155 ET, 64 PMF and three unclassifiable MPNs (MPN-U) (for clinical features refer to Table S1, and for frequency distribution of MPN driver mutations to Figure S1A). By including in this analysis the first 115 nucleotides of CALR 3′-UTR,6 not always covered by conventional diagnostic methods, we identified CALR mutations in 48 (17%) out of 286 MPNs, comprising a case of double positive JAK2V617F/CALR and five previously unreported CALR exon 9 mutations (Table S2).
Two noncanonical CALR mutations, c.1254 + 10_ + 33del24 (CALR del24) and E405_D408-del c.1214_1225del12 (CALR del12), were identified in blood cells from three patients (patients #1, #2, and #3) with a 2016 WHO diagnosis of MPN-U. These cases lacked JAK2 mutations but presented clinical, laboratory and histological features recalling PV (Figure 1A-C, Table S3, Figure S1B, and case reports in Appendix S1). Peripheral blood mononuclear cells (PBMCs) from patients#1, #2, and #3 were hypersensitive to erythropoietin (EPO)-induced production of erythroid colonies in vitro (Figure 1D). Figure 1D shows that at very low EPO concentrations (0.1 IU/mL), BFU-E growth was intermediate between that of four healthy donor samples and a control group of 8 JAK2V617F-mutated PVs. Moreover, an impairment in the number of CFU-GM colonies was also measured. Morphologic assays performed after 14 days of EPO 3 IU/mL culture, showed a predominance of mature erythroblasts (orthochromatic) in BFU-E colonies from patients #1, #2, #3. By contrast, erythroblasts at earlier maturation stages (proerythroblasts and basophilic erythroblasts) were present in BFU-E colonies from healthy donors (Figure 1E).
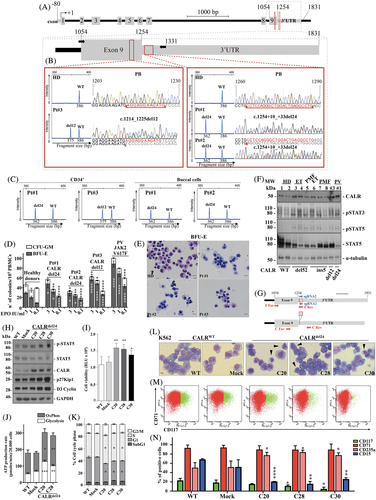
Patients #1, #2 are siblings and carried the previously unreported CALR del24, which, interestingly, does not affect protein sequence but disrupts the proximal region of 3′-UTR mRNA commonly converted into coding sequence by CALR mutations in MPNs (Figure 1A, Table S2). Patient #3 displayed the in-frame del12, causing the loss of four negatively charged amino acids (405-408 aa, stretch IV in Table S2) and encoding a novel CALR protein maintaining KDEL unaltered (Figure 1A, Table S2). Note, del12 was previously described in PMFs and found in a JAK2V617F+ PMF case of our cohort (Table S2). Notably, CALR del24 and del12 occur in evolutionarily conserved CALR gene regions. Neither of these mutations affect the integrity of the KDEL motif, but are predicted to cause structural variations at the proximal CALR 3′-UTR mRNA, commonly converted into coding sequence by CALR frameshift mutations (Figure 1A, Figure S2, Table S2).
Polymerase chain reaction followed by fragment length analysis6 estimated a CALR mutant allele burden ≥50% in PB granulocytes of patients #1, #2, and #3 (Figure 1B), whereas the median mutant allele burden of CALR-mutated ET or PMF patients was 33% and 44%, respectively (Figure S1C). Both del24 and del12 on CALR exon 9 were also detected in CD34 + -hematopoietic cells from patients #1, #2, and #3, supporting their occurrence in early progenitors and clonal hematopoiesis, with mutant allele burdens ≥50% (Figure 1B,C and data not shown). Note, CALR del24 with a ≥ 50% mutant allele frequency was also detected in both siblings' buccal cell DNA, suggesting that such mutation is inherited (Figure 1C). Unfortunately, additional samples from patients and their relatives were unavailable for further investigations.
A targeted NGS analysis7 on granulocyte DNA from these patients confirmed the presence of the del24 or the del12 on CALR exon 9 and excluded alterations of genes commonly involved in rare inherited erythrocytosis or thrombocytosis (Table S4). Moreover, the siblings exhibited del24 in CALR 3′-UTR with a > 45% variant allele frequency (VAF), and displayed mutations in ASXL1 (patient #1) or DNMT3A (patient #2), two complementary molecular clonal markers of myeloid neoplasms known to lead to clonal hematopoiesis (Table S5).
Recurrent CALR deletions/insertions of MPN, cause a frameshift, modifying the protein sequence.2, 3 Instead, CALR del24 affects only 3′-UTR mRNA, whereas CALR del12 partially alters the coding sequence, but does not affect KDEL. Therefore, CALR del24 and del12 mutants lack the key molecular determinants constitutively activating the MPL-JAK/STAT signaling pathway, which underlies ET- and PMF-like phenotypes in cellular/animal models1 (and references therein).
Nevertheless, immunoblot analysis showed that, with respect to healthy donor samples and similarly to CALR-mutated ET and PMF, increased phosphorylation status of STAT3 and STAT5 is detectable in granulocytes isolated from CALR del24 and CALR del12 cases, suggesting an activation of JAK/STAT signaling (Figure 1F). Moreover, granulocytes from these cases expressed CALR at the highest levels, a finding consistent with the disruption of 3′-UTR mRNA sequence.4
To address the biological consequences of structural variations in CALR 3′-UTR, using the CRISPR-Cas9 technology, we introduced the CALR del24 mutation within CALR 3′-UTR in K562 cells, which are able to differentiate along erythroid or megakaryocytic lineages, despite the expression of BCR/ABL (Figure 1G, Appendix S1). The CALR genome matched sites and potential off-targets are shown in Table S6. The K562 C20, C28 and C30 clones, carrying CALR del24 in 3′-UTR, were selected by PCR (Figure S3A). Efficiency of gene editing was evaluated by Sanger sequencing and fragment length analysis (Figure S3B), as reported in Appendix S1. The three clones displayed the mutation c.1254 + 10_ + 33del24, besides partial deletions of the targeted region on the other CALR allele. Mutant allele burdens in K562 C20, C28 and C30 mutated clones were respectively of 57%, 54% and 45%, thus very similar to those measured in patients #1, and #2 primary blood cells (Figure S3B, Figure 1B,C). The CALR mRNA and protein levels were increased in CALR mutated clones, whereas BCR/ABL1 (e14a2) expression was unaffected (Figure 1H, Figure S3C,D).
When compared to K562 control cells, pSTAT5 was induced in CALR mutated clones, supporting an active JAK/STAT signaling in these cells (Figure 1H). Moreover, these clones showed increased cell viability (Figure 1I), glycolytic activity and mitochondrial oxidative phosphorylation (Figure 1J, Figure S3E-H), promotion of G1/S phase transition of the cell cycle and increased levels of cyclin D3 and p27Kip1 (Figure 1 H,K). These are all well recognized biological features of JAK2-mutated PVs. Importantly, CALR del24 also induced morphological changes in K562 cells, including cell size reduction, chromatin condensation, decreased cytosolic basophilia, appearance of mature erythroblasts, which are consistent with their maturation toward erythropoiesis (Figure 1L). Moreover, flow cytometric analysis showed a significant decrease of the percentage of CD117 + -erythroid precursors, while the percentage of CD235a/glycophorin A+ − differentiated erythroid cells increased and CD71 + -pro-erythroblasts remained at the levels measurable in K562 wild-type and mock cells (Figure 1M,N). A significant decrease in the percentage of CD15 + -granulocytic cells in CALR mutated K562 clones was also observed.
In conclusion, we report the identification of noncanonical mutations affecting CALR 3′-UTR in JAK2 mutation-negative patients with MPN diseases resembling PV. These mutations occur in evolutionarily conserved CALR regions and are predicted to disrupt mRNA secondary structure of the proximal CALR 3′-UTR region, which commonly generates a novel peptide through the frameshifts of canonical CALR deletions/insertions. Notably, the disruption of this CALR-3′-UTR region in myeloid cells using CRISPR-Cas9 genome-editing induces well recognized biological features of PVs. If a deletion in proximal CALR 3′-UTR can determine such phenotypic changes, we hypothesize that this noncoding mRNA region has hematopoietic functions, thus its disruption might affect PV onset. Functional roles of 3′-UTRs in hematopoietic lineage development and differentiation are emerging5 and 3′-UTR mRNA structural variations have been related to cancer.4 Whereas further studies addressing the mechanisms underlying these findings are needed, the systematic screening of CALR 3′-UTR region might reveal novel aspects of MPN pathogenesis and clarify if our cases are a rare finding or a recurrent genotype ignored so far.
ACKNOWLEDGMENTS
We are most grateful to all the patients and the clinicians of the Hematology Unit of Santa Maria Goretti Hospital, Latina, Italy for making this study possible. In addition, we thank Dr. Fabrizio Padula for assistance with cell cycle and apoptosis analyses, Professor Stefano Ascani and Dr. Giovanni Martino of the Pathology Unit, Hospital of Terni and Università degli Studi di Perugia, Italy, for their careful independent re-evaluation of morphologic parameters in BM specimens from the MPN-unclassifiable (MPN-U) cases. Work at CRIMM (Florence) was supported by AIRC 5 x 1000 “Metastatic disease: the key unmet need in oncology” to MYNERVA project, #21267 (MYeloid NEoplasms Research Venture AIRC). Funding for the project was provided by the University of Roma “La Sapienza” (RM11715C81899055 and RM11816436B700D1) to CN and GC. This work was supported in part by the Associazione Italiana per Ricerca sul Cancro (AIRC-IG-11949 to CN) and Fondazione Cassa di Risparmio di Perugia to FG.




