The clinical impact of time to response in de novo accelerated-phase chronic myeloid leukemia
Funding information: MD Anderson Cancer Center Support Grant, Grant/Award Number: P30CA016672
Abstract
We aimed to describe the impact of time to response on the outcomes of 75 patients with accelerated-phase chronic myeloid leukemia (CML-AP) at diagnosis. Patients had at least 1 feature of AP: blasts ≥15% (n = 2), basophils ≥20% (n = 19), platelets <100 × 109/L (n = 7), cytogenetic clonal evolution (n = 34), or more than one factor (n = 13). Thirty-three patients received imatinib; 42 received a second-generation tyrosine kinase inhibitor (2GTKI) (19 dasatinib and 23 nilotinib). We used chi-square and Kaplan-Meier analyses to determine the impact of various degrees of molecular and cytogenetic response at early time points (3 and 6 months) on rates of overall cytogenetic and molecular response, overall survival (OS), event-free survival (EFS), transformation-free survival (TFS), and failure-free survival (FFS). After a median follow-up of 96 months (range: 18-224 months), the overall rate of complete cytogenetic response was 79%, of major molecular response, 71%, and of molecular reponse (MR)4.5, 59%. Patients who achieved a major cytogenetic response (MCyR) (n = 49) at 3 months had significantly better 3-year OS (94% vs 75%; P = .002), TFS (98% vs 73%; P < .001), EFS (93% vs 42%; P < .001), and FFS (83% vs 25%; P < .001) rates than patients who did not have MCyR at 3 months. Most (67%) who eventually achieved sustained MR4.5 had achieved MCyR at 3 months. In de novo CML-AP, early response at 3 and 6 months is a strong determinant of long-term outcome.
1 INTRODUCTION
Although most patients with chronic myeloid leukemia (CML) are in the chronic phase (CML-CP) at the time of diagnosis, approximately 5% to 10% present with features of accelerated-phase CML (CML-AP).1 Imatinib, dasatinib, nilotinib and bosutinib are the standard initial tyrosine kinase inhibitors (TKIs) for patients with CML-CP, but there is only limited data on their efficacy in patients with de novo CML-AP, a clinical presentation which is itself uncommon.2, 3 Most previous studies focused on patients whose disease progressed to the accelerated phase after previous therapies failed. Previous studies have shown that patients with CML-AP features at the time of diagnosis may have a favorable outcome, similar to that of patients with CML-CP, when treated with TKI, particularly with second generation agents.3
Among patients with CML-CP, time to response is an important determinant of outcome,4, 5 a fact that is prominently featured in the ELN and other recommendations.6 However, the clinical impact of early response for patients with de novo accelerated-phase CML has not yet been evaluated. Thus, the purpose of this analysis was to assess the clinical impact of time to response in patients with CML-AP at diagnosis who were treated with single-agent first-generation or second-generation TKIs (2GTKI) as initial therapy.
2 METHODS
2.1 Patient population
We conducted a retrospective analysis of 75 adult (age ≥18 years) patients with confirmed de novo CML-AP who were treated with TKIs as initial therapy at our institution on parallel or consecutive prospective clinical trials (n = 42) or off protocol (n = 33) from September 1999 through November 2016. The trials had similar designs, eligibility criteria, and monitoring protocols (NCT00129740 NCT00254423, NCT02689440). Patients treated outside of clinical trials were monitored with a similar schedule as the protocols and were studied in a retrospective chart review. Eligible patients had any of the following features of CML-AP: 15%-29% blasts in peripheral blood (PB) or bone marrow (BM); blasts + promyelocytes ≥30% in PB or BM; basophils ≥20% in PB or BM; platelets <100 × 109/L unrelated to therapy; and/or cytogenetic clonal evolution.1 This definition of CML-AP is the standard definition that has been used in all pivotal studies of TKIs.3 Other inclusion criteria were Eastern Cooperative Oncology Group performance status of 0 to 2 and acceptable end organ function, including total bilirubin <1.5× upper limit of normal (ULN), alanine aminotransferase <2.5× ULN, and creatinine <1.5× ULN. A negative pregnancy test was required for inclusion of women of childbearing potential. Two of the 33 patients treated off protocol did not meet the eligibility criteria: one had a mild elevation in total bilirubin [30.8 μmol/L] and one had mild elevation in creatinine [159 μmol/L]. Patients must have initiated treatment within 1 year of diagnosis of CML-AP and, except for hydroxyurea, could not have received more than minimal therapy, that is, less than 1 month of prior interferon-alpha and/or TKI exposure.
Written informed consent was obtained from all patients in accordance with institutional guidelines. A waiver for informed consent was granted by the institutional review board for patients enrolled in the retrospective chart review. The trial and chart review protocols were approved by the Institutional Review Board at MD Anderson Cancer Center and were executed in adherence with the principles of the Declaration of Helsinki.
2.2 Cytogenetic and molecular testing
The patients underwent cytogenetic analysis (assessed by PB and/or BM) every 3 months for the first year and every 6 to 12 months thereafter. Molecular testing for BCR-ABL1 fusion transcripts using real-time polymerase chain reaction (PCR) was generally conducted every 3 months for the first year and every 6 months thereafter. However, 27 patients (three who received 2GTKIs and 24 who received imatinib) who were enrolled and treated on earlier protocols (before 2002) did not have molecular testing data available for the first several months of treatment. That is because molecular testing was not routinely done at that time until achievement of cytogenetic response, or did not produce quantitative data.
2.3 Response criteria
Standard response criteria for CML were used as previously described.6, 7 Briefly, complete hematologic response (CHR) included normalization of the blast percentage in PB and BM (≤5% BM blasts); leukocytes <10 × 109/L; resolution of signs and symptoms of CML; normalization of PB differential (with no peripheral blasts, promyelocytes, or myelocytes); and platelet count <450 × 109/L. When thrombocytopenia (platelets <100 × 109/L) was present before treatment, platelet count normalization to a level > 100 × 109/L was necessary for classification of the response as CHR. Patients who had a normal platelet count before initiating treatment, but who later developed thrombocytopenia while on therapy could be considered to have CHR if all other criteria for CHR were met.6, 7
Conventional cytogenetic analysis of 20 metaphases was used for evaluation of cytogenetic response (assessed by PB and/or BM). Cytogenetic responses were categorized as no response if the percentage of Philadelphia chromosome-positive (Ph+) metaphases was 36% to 100%, partial cytogenetic response (PCyR) if 1% to 35%, and complete cytogenetic response (CCyR) if 0%. Patients with either CCyR or PCyR were considered to have a major cytogenetic response (MCyR) (≤ 35% Ph + metaphases).6
The BCR-ABL transcripts were measured by quantitative real-time PCR analysis of PB and/or BM aspirate samples.8 Major molecular response (MMR) was defined as ≤0.1% BCR-ABL1/ABL1 in the International Scale (IS). Molecular response (MR)4 was defined as BCR-ABL1/ABL1 transcripts <0.01%, and MR4.5 was defined as BCR-ABL/ABL1 ≤ 0.0032% IS4).6, 9
2.4 Survival outcomes
Event-free survival (EFS) was measured from the date of the start of therapy to the occurrence of any of the following events: loss of CHR, loss of MCyR, transformation, death while on study, or an increase in white blood cell count. Transformation-free survival (TFS) was determined from the date of the start of therapy to the date of transformation to blast-phase CML or death while on study. Overall survival (OS) was calculated from the date of the start of therapy to the date of death from any cause at any time or last follow-up. Failure-free survival (FFS) was calculated from the date of the start of therapy to the date of treatment failure. This was defined as the first instance of any of the following: any of the aforementioned criteria for events; loss of CCyR; lack of MCyR by 12 months; lack of CCyR by 18 months; development of resistant ABL mutations; noncompliance that led to discontinuation; development of toxicity or intolerance; or permanent discontinuation for any reason including a switch to a different TKI. Patients who had a sustained molecular response of MR4.5 (for at least 2 years) and opted to discontinue all TKI treatment with regular response monitoring, were censored at the time of discontinuation, but this was not considered a failure-defining event.
2.5 Statistical analysis
The chi-square test was used to analyze categorical variables. Survival probability estimates were made using the Kaplan-Meier method and survival estimates compared using the log-rank test.
3 RESULTS
3.1 Patient characteristics
Table 1 summarizes the clinical and demographic characteristics of the 75 patients included. The median age was 48 years (range, 22-81 years). Most of the patients had only one feature of CML-AP at initial diagnosis, but 13 (17%) patients had more than one. The most common features were increased basophil counts (n = 19, 25%) and cytogenetic clonal evolution (n = 34, 45%). The presence of 15%-29% blasts was noted in two (3%) patients and seven (9%) patients had platelets <100 × 109/L.
| Characteristics | n (%) or median [range] | P value | |||
|---|---|---|---|---|---|
| Overall (n = 75) | 2GTKIa (n = 42) | Imatiniba (n = 33) | |||
| Age (y) | 48 [22-81] | 49 [23-80] | 46 [22-81] | .970 | |
| Male | 39 (52) | 23 (55) | 16 (48) | .589 | |
| Time from diagnosis to TKI initiation (mo) | 0.7 [0-5.9] | 0.3 [0-2.5] | 0.9 [0-5.9] | .003 | |
| CML-AP feature | Basophils ≥20% | 19 (25) | 9 (21) | 10 (30) | .379 |
| Clonal evolution | 34 (45) | 19 (45) | 15 (45) | .984 | |
| Blasts 15%-29% | 2 (3) | 1 (2) | 1 (3) | .865 | |
| Platelets <100 × 109/L | 7 (9) | 5 (12) | 2 (6) | .390 | |
| >1 factor | 13 (17) | 8 (19) | 5 (15) | .660 | |
- Abbreviations: CML-AP, accelerated phase of CML; 2GTKI, second-generation TKI; TKI, tyrosine kinase inhibitor.
- a Doses of TKI: imatinib: 400 mg (n = 8, 11%); ≥600 mg (n = 25, 33%); dasatinib: 50 mg (n = 2, 3%); ≥100 mg (n = 17, 23%); nilotinib: 600 mg (n = 1, 1%); 800 mg (n = 22, 29%).
Among the 75 patients included, 33 (44%) received imatinib as initial therapy, most at a starting dose of at least 600 mg/d (n = 25, 33%) (Table 1). Among patients receiving imatinib at a dose higher than 600 mg/d, one received 500 mg twice a day and seven received 800 mg/day. Most (n = 23, 70%) of the 33 imatinib-treated patients started therapy before 2002, when cytogenetic and molecular testing were not routinely performed at early monthly response evaluations. Among the patients treated with 2GTKI, 23 received nilotinib and 19 received dasatinib. Most patients started TKI treatment within 1 month of initial diagnosis, with a median time to treatment of 0.7 months (range, 0-5.9 months). Patients who received 2GTKIs initiated treatment approximately 2.5 weeks sooner than patients who received imatinib (P = .003).
3.2 Effect of early response on overall response
The median follow-up for the total population is 96 months (range, 18-224 months). Because imatinib was available several years earlier, the median follow-up time for patients treated with 2GTKI was significantly shorter than for patients treated with imitinib (imatinib, 184 months vs 2GTKIs, 79 months; P < .001). The best overall responses and survival probabilities are shown in Table 2. Overall, 79% of patients (81% on 2GTKI and 76% on imatinib) achieved CCyR. Overall, 70% of patients (79% in the 2GTKI group and 59% in the imatinib group; P = .073) achieved MMR, and 58% attained MR4.5 (62% on 2GTKI and 53% on imatinib; P = .522).
| Response/survival measure | Overall (N = 75) (73 evaluable for CG; 74 for MR) | 2GTKI (N = 42) | Imatinib (N = 33) (32 evaluable for MR) | P value | ||
|---|---|---|---|---|---|---|
| Median [range] follow-up, mo | 96 [18–224] | 79 [18-149] | 184 [5-224] | <.001 | ||
| Best overall response, n (%) or median [range] in mo | MCyR | 61 (81) | 34 (81) | 27 (82) | .928 | |
| Time to MCyR | 3 [1-12] | 3 [1–12] | 4 [2–8] | .486 | ||
| CCyR | 59 (79) | 34 (81) | 25 (76) | .589 | ||
| Time to CCyR | 3 [2–20] | 3 [2–18] | 4 [2–20] | .439 | ||
| MMR | 52 (70) | 33 (79) | 19 (59) | .073 | ||
| Time to MMR | 6 [3-99] | 6 [3–99] | 10 [3-26] | .104 | ||
| MR4 | 46 (62) | 27 (64) | 19 (59) | .667 | ||
| Time to MR4 | 8 [3-77] | 8 [3–77] | 16 [3-53] | .300 | ||
| MR4.5 | 43 (58) | 26 (62) | 17 (53) | .447 | ||
| Time to MR4.5 | 12 [3-93] | 12 [3-93] | 17 [6-53] | .434 | ||
| Survival probability | OS | Deaths | 18 | 6 | 12 | .099 |
| 3 y | 86% | 95% | 76% | |||
| Median (mo) | NR | NR | NR | |||
| TFS | Transformations | 7 | 3 | 4 | .498 | |
| 3 y | 90% | 92% | 89% | |||
| Median (mo) | NR | NR | NR | |||
| EFS | Events | 21 | 10 | 11 | .469 | |
| 3 y | 77% | 82% | 71% | |||
| Median (mo) | NR | NR | NR | |||
| FFS | Failures | 37 | 16 | 21 | .105 | |
| 3 y | 62% | 70% | 53% | |||
| Median (mo) | 89 | NR | 39 | |||
- Abbreviations: CCyR, complete cytogenetic response (0% Ph + metaphases); CG, cytogenetic response; EFS, event-free survival; FFS, failure-free survival; MCyR, major cytogenetic response (≤35% Ph + metaphases); MMR, major molecular response (≤ 0.1% BCR-ABL1/ABL1 in the International Scale [IS]); MR, molecular response; MR4 (BCR-ABL/ABL transcripts <0.01%); MR4.5 (BCR-ABL/ABL1 ≤ 0.0032% IS4); NR, not reached; OS, overall survival; TFS, transformation-free survival.
We then analyzed the long-term outcome according to early response assessments. Because in earlier years molecular monitoring was not done routinely until patients achieved cytogenetic response, most imatinib-treated patients did not have a PCR performed at 3 months. We thus analyzed the data by achievement of MCyR (which is grossly equivalent to a BCR-ABL1/ABL1 level of ≤10%). For this analysis we used the optimal response criteria established for CML-CP.6 We also performed the analysis using molecular response at these same timepoints on an intention-to-treat analysis. For this purpose we considered anyone on therapy who had no quantitative PCR data at the given timepoint as not having achieved an early molecular response (ie, considered them not to have ≤10% BCR-ABL1/ABL1 by IS), as we could not confirm they had transcript levels below this threshold.
The overall outcomes according to early response are summarized in Table S1. At 3 months 73 of the 75 patients were evaluable for cytogenetic response and 72 for molecular response; the two inevaluable patients came off of frontline nilotinib at 0.33 months (due to grade 4 thrombocytopenia and died 4 days later of unknown causes), and 0.46 months (due to lack of insurance coverage; transitioned to imatinib). One patient who was only evaluable for cytogenetic response at 3 months was inevaluable for molecular response due an unquantifiable transcript (the BCR-ABL subtype e1a2). Similarly, at 6 months, 71 patients were evaluable for cytogenetic response and 70 for molecular response. The four inevaluable patients for cytogenetic and molecular response included the two who were inevaluable at 3 months, one patient who progressed to CML-BP at 2.27 months (while on nilotinib), and one who discontinued imatinib at 2.5 months due to toxicity (myelosuppression and rash). The one additional patient who was inevaluable for molecular response was the patient with e1a2 mentioned above.
At 3 months 49/73 patients (67%) patients had achieved a MCyR. Compared to patients with no MCyR at 3 months, a significantly higher percentage of patients who had at least MCyR at 3 months eventually attained CCyR (MCyR: 96% vs no MCyR: 50%; P < .001). Patients with at least MCyR at 3 months also had a significantly higher probability of later achieving MMR (P < .001) and MR4.5 (P < .001) than did patients with no MCyR at this time point. Having a PCR <10% at 3 months was associated with higher overall rates of CCyR (P = .006), MMR (P = .002), and MR4.5 (P = .023). At 6 months, PCR < 10% was associated with higher rates of CCyR (P = .009) and MMR (P < .001) at any time (Table S1).
3.3 Effect of early response on sustained molecular responses
Of the 49 patients who achieved MCyR at 3 months, 39 received therapy for at least 2.5 years, thus considered evaluable for achievement of sustained MR4.5. Among them, patients who achieved MCyR by 3 months had a higher probability of eventually achieving a sustained MR4.5 than did those who did not achieve MCyR by 3 months (Table S1). Sixty-seven percent (26/39) of patients who were evaluable for sustained MR4.5 had achieved MCyR at 3 months vs 29% (2/7) of patients lacking MCyR at 3 months (P = .057). All 12 patients who had >35% Ph + at 6 months had changed TKI therapy before 2.5 years on therapy; therefore, none achieved a sustained MR4.5 on initial therapy. The change of therapy was because of loss or failure to achieve CHR in five patients (two had concurrent toxicity), progression to blast phase in three, non-compliance in three and toxicity in one patient. There were no significant differences in the rates of sustained MR4.5 among patients with PCR <10% vs >10% at 3 or 6 months.
3.4 Effect of achieving MCyR at 3 months and maintaining MCyR at 6 months vs not achieving MCyR until 6 months
We then examined the outcomes of patients who were evaluable for MCyR at both 3 and 6 months (Table S2A and S2B). Of the 49 patients who achieved a MCyR by 3 months, 48 maintained or improved MCyR at 6 months (Table S2B); one patient had lost the MCyR at 6 months with a rapid transformation to blast phase.
Of the 24 patients who did not achieve MCyR by 3 months, 22 patients remained on therapy at 6 months; the remaining two patients discontinued their initial TKI therapy prior to 6 months because of toxicity (rash and myelosuppression; n = 1), or progression to blast phase followed by death (n = 1). Compared to patients who had not achieved MCyR by either 3 or 6 months (n = 11), those evaluable patients who did not achieve MCyR by 3 months, but achieved MCyR by 6 months, had a greater overall probability of achieving CCyR (P < .001), MMR (P = .023), MR4 (P = .023), and MR4.5 (P = .023) (Table S2A).
We also examined whether achieving MCyR at 3 months and maintaining MCyR at 6 months resulted in better long-term outcomes than not achieving MCyR until 6 months. Patients who already achieved MCyR by 3 months had a significantly greater probability of eventually attaining MMR (P < .001), MR4 (P = .004), and MR4.5 (P = .023) (Table S2B).
3.5 Effect of early response on survival outcomes
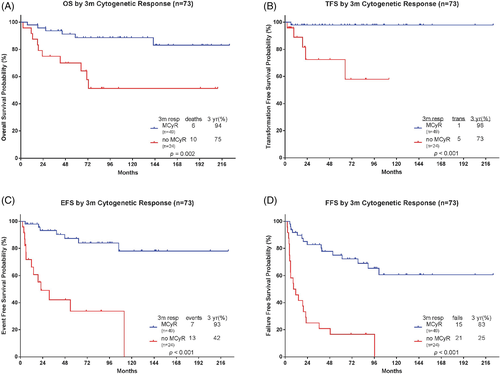
We next looked at the impact of early response on long-term survival outcomes. Patients who achieved MCyR (n = 49) at 3 months had a significantly higher 3-year OS probability than did patients without MCyR (n = 24) at 3 months (94% vs 75%; P = .002) (Figure 1A). The causes of death are summarized in Table S3. Similarly, patients who had MCyR at 3 months also had significantly higher probabilities of TFS (98% vs 73%; P < .001), EFS (93% vs 42%; P < .001), and FFS (83% vs 25%; P < .001) than did patients who did not have MCyR at 3 months (Figure 1B-D, Table S4). Patients who achieved MCyR (n = 59) at 6 months also had a significantly higher 3-year OS, TFS, EFS, and FFS probability.
By intention to treat (ITT) analysis, when examining patients evaluable for PCR at 3 months (n = 72), 39/72 (54%) had PCR < 10%, and these patients had significantly higher 3-year OS (95% vs 79%; P = .019), TFS (97% vs 84%; P = .036), EFS (94% vs 59%; P = .001), and FFS (81% vs 45%: P = .001) than did patients with PCR >10% at 3 months. When examining patients evaluable for PCR at 6 months (n = 70), 46/70 (66%) had PCR <10%, and these patients had a significantly higher 3-year EFS (84% vs 67%; P = .047) and FFS (72% vs 54%; P = .053) than did patients with PCR >10% at 6 months; however, the impact was not statistically significant for OS and TFS (Table S4).
Patients who achieved MCyR (n = 59) at 6 months also had a significantly higher OS probability than did patients without MCyR (n = 12) at 6 months (95% vs 67%; P < .001) (Figure S1A) at 3 years. Similarly, patients who had MCyR at 6 months had significantly higher 3-year TFS (98% vs 0%; P < .001), EFS (90% vs 0%; P < .001), and FFS (76% vs 0%; P < .001) probabilities than did patients without MCyR at 6 months (Figure S1B-1D).
Only four patients progressed to CML-BP (three myeloid and one lymphoid) while on frontline TKI for de novo CML-AP over a median time of 8 months (2.27-22.14 months). One patient progressed to CML-BP rapidly at 2.27 months. The other three had failed to achieve MCyR at 3 and 6 months, and progressed to BP at 6.4, 9.5 and 22.1 months, respectively.
3.6 Effect of TKI type on response and survival outcomes
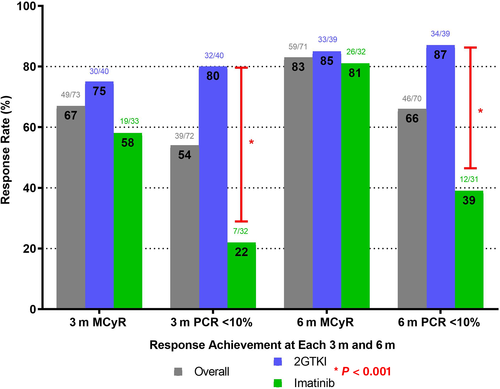
As shown in Table S5, the probability of achievement of MCyR, CCyR, MR,4 and MR4.5 at 3 and 6 months was similar for patients receiving imatinib and 2GTKI. While there were no significant differences between rates of MMR at 3 months between patients receiving imatinib vs 2GTKI at 6 months, patients receiving 2GTKI had a higher rate of MMR (P = .028). Patients on 2GTKI experienced higher rates of PCR <10% at 3 months and 6 months (Figure 2). Among patients with a similar early response at each time point, the specific TKI with which the given response was achieved had no impact on the survival endpoints (OS, TFS, EFS, and FFS) (Figures S2A-D, S3A-3D).
3.7 Outcomes and response rates by MCyR at 3 months vs 6 months
There was no significant difference in OS between patients who achieved MCyR at 3 months and those who failed to achieve MCyR at 3 months but achieved MCyR at 6 months (Table S2A). However, compared to patients who had not achieved MCyR by either 3 or 6 months (n = 11), those evaluable patients who did not achieve MCyR by 3 months but did achieve MCyR by 6 months had superior rates of FFS (P < .001), EFS (P < .001), and TFS (P = .007) (Table S2A).
Compared to those patients who did not achieve MCyR by 3 months but did achieve MCyR by 6 months, having attained MCyR faster by 3 months was associated with superior rates of FFS (P = .004), EFS (P = .005), and TFS (P = .003) (Table S2B). Of the 18 deaths observed so far, seven were due to blast phase (four of whom progressed to CML-BP on their initial TKI and three on subsequent therapy) (Table S3).
Among the 24 patients who failed to achieve MCyR at 3 months, 22/24 remained on therapy at 6 months (2/24 patients discontinued their initial TKI therapy prior to 6 months because of toxicity [n = 1] or progression to blast phase followed by death [n = 1]). Eleven out of 22 patients achieved MCyR by 6 months. The remaining 11 patients who did not achieve MCyR by 3 or 6 months overall did poorly on frontline TKI therapy, with a median time on initial TKI of only 6 months (4-23 months). Only 1/11 patients achieved MCyR at 12 months and CCyR by 18 months, but progressed to CML-BP while on study at 22 months. The remaining 10/11 patients never achieved MCyR, CCyR, MMR, or MR4.5 on their frontline TKI.
Among the 10 patients who never responded to frontline TKI, 4/10 died (all shown in Table S3): 3/4 who died had undergone stem cell transplantation after failing frontline TKI; 1/4 progressed to CML-BP while on frontline TKI, which was followed by chemotherapy with no response. Six of the 10 patients who never achieved MCyR, CCyR, MMR, or MR4.5 on their frontline TKI are still alive. The first patient subsequently received DCC-2036 but failed to achieve MCyR so underwent allogeneic stem cell transplantation, achieving CCyR with BCR-ABL negativity by PCR. The second patient received bosutinib, attaining CCyR and MMR, but later lost the cytogenetic response, and at last follow-up had also failed subsequent lines of therapy (ponatinib and combination chemotherapy combined with ponatinib and venetoclax). The third patient proceeded to allogeneic stem cell transplantation and achieved CCyR with undetectable BCR-ABL by PCR. The fourth patient received subsequent therapy with decitabine + dasatinib, but failed to achieve a cytogenetic response and underwent allogeneic stem cell transplantation resulting in a CCyR. The patient received posttransplant ponatinib for persistently detectable BCR-ABL by PCR. After attaining undetectable BCR-ABL, the patient was transitioned to imatinib due to ponatinib-related toxicity; BCR-ABL remains undetectable by PCR. The fifth patient proceeded to allogeneic stem cell transplantation with undetectable BCR-ABL by PCR, and the sixth patient was lost to follow-up.
4 DISCUSSION
Note, CML-AP most frequently represents disease progression: it follows the chronic phase and precedes the terminal blast phase. Characterized by cytogenetic instability and progressive impairment of myeloid cell differentiation, CML-AP has an aggressive clinical course and historically has been associated with a median survival time of only 18 to 36 months.1, 8, 10 However, patients who have features of AP at the time of diagnosis have a better prognosis, particularly when treated with TKIs. Those who receive second generation TKI have a clinical course akin to patients diagnosed in CP.3 In view of this parallel, we investigated whether the early response criteria used to assess and predict outcome in CML-CP5, 11 apply also to patients diagnosed in AP. The results of our analysis suggest that among patients with clinical features of CML-AP at the time of diagnosis who are treated with TKI, early response at 3 and 6 months correlates with long-term outcome, much as has been reported for patients with CML-CP.
Our analysis shows that the OS, EFS, TFS, and FFS probabilities for patients who achieved MCyR at 3 months were significantly superior to those for patients who did not achieve such responses. These differences were amplified when assessed based on the response achieved at 6 months. With the caveats of comparison across different reports, the differences in long-term outcome between early responders and non-early responders seem to be greater, for patients in CML-AP in this report than what has been reported in CP. For example, in a similar analysis, we reported 3-year OS for patients with early MCyR at 3 months vs non responders among patients in chronic phase of 96% vs 92%,12 whereas in the CML-AP analysis reported here, the OS probability is 94% vs 75% (P = .002). Corresponding rates for EFS were 89% vs 81% for patients in CP12 and 93% vs 42% (P < .001) in AP. In this analysis, we also observed that the majority of patients (67%) with sustained MR4.5 had achieved MCyR at 3 months, further underscoring the clinical significance of early response in the AP population.
There has been much debate on the best course of action and the timing of such action for patients with lack of early response to TKI in CML-CP. There was a recent randomized trial of patients with suboptimal response/warning at 3 months while on therapy with imatinib. It showed that patients who switched to dasatinib had a greater probability of achieving an MMR by 12 months, when switched to dasatinib compared to maintaining therapy with imatinib. There was, however, no difference in OS or PFS at the time of the analysis. Whether there are later benefits with such switch in the long-term outcomes will require longer follow-up for this study.13 The observation in our report of the larger difference between patients with and without early molecular response supports considering switching TKIs, on the basis of the results of the 3-month evaluation, instead of waiting until the 6-month evaluation as is current practice.14 Still clinical trials are required to confirm the value of such intervention.
In conclusion, patients with features of the accelerated phase at the time of CML diagnosis have excellent outcomes when treated with TKIs as initial therapy, particularly with 2GTKIs. The outcomes for patients with similar favorable responses at early time points are similar regardless of the TKI used. Further studies are needed to assess the proper approach for patients with CML in accelerated phase with suboptimal response at 3 months.
ACKNOWLEDGMENTS
This research was supported in part by the MD Anderson Cancer Center Support Grant P30CA016672. We acknowledge the Department of Scientific Publications for critical review of this manuscript.
AUTHOR CONTRIBUTIONS
M.O. collected data and wrote the manuscript. H.K. reviewed and approved the manuscript. M.S. collected data. S.D. collected data and performed response and survival analysis. A.M. collected data. G.M.N.G. reviewed and approved the manuscript. E.J. reviewed and approved the manuscript. L.A. performed cytogenetic analysis. S.V. reviewed and approved the manuscript. G.B. reviewed and approved the manuscript. F.R. reviewed and approved the manuscript. G.G.M. reviewed and approved the manuscript. G.T. reviewed and approved the manuscript. R.C. reviewed and approved the manuscript. S.P. collected data and performed response and survival analysis. A.F. reviewed and approved the manuscript. T.K. reviewed and approved the manuscript. J.C. wrote, reviewed, and approved the manuscript.
CONFLICT OF INTERESTS
M.O.: none; H.K.: Research grants from Novartis, BMS, Ariad, Pfizer, AbbVie, Agios, Amgen, Astex, Cyclacel, Daiichi-Sankyo, Immunogen, Jazz Pharma; M.S.: none; S.D.: none; A.M.: none; G.M.N.G.: none; E.J: Consultancy honoraria from BMS, Novartis, Pfizer, and Ariad; Research support from Amgen, Abbvie, Spectrum, BMS, Takeda, Pfizer and Adaptive. L.A.: none; S.V.: none; G.B.: Advisory board Novartis, Honaria from: Sun Pharma; F.R.: Honoraria from BMS, Novartis, Pfizer; G.G.-M.: none; G.T.: none; R.C.: none; S.P.: none; A.F.: none; T.K.: none; J.C.: Research support (to my institution) from BMS, Novartis, Takeda, Chemgenex, Sun Pharma and Pfizer. Consultancy: Pfizer, Takeda, Novartis.




