A population-based study of chronic eosinophilic leukemia-not otherwise specified in the United States
Chronic eosinophilic leukemia-not otherwise specified (CEL-NOS) is a rare myeloproliferative neoplasm (MPN) characterized by clonal proliferation of eosinophil precursors and accompanying end-organ damage - either due to leukemic infiltration or mediated by cytokines, enzymes, or other proteins The diagnosis of CEL-NOS necessitates the demonstration of persistent eosinophilia involving the peripheral blood (≥1.5 × 109/L) and bone marrow, an implicated cytogenetic or molecular abnormality or increased blasts, and the absence of fusion transcripts (BCR-ABL1 fusion, PDGFRA, PDGFRB, or FGFR1; PCM1-JAK2, ETV6-JAK2, or BCR-JAK2 fusion), and diagnostic criterion for other myeloproliferative states.1 Given the rarity, no consensus in treatment exists, with implemented therapeutics including hydroxyurea, steroids, imatinib, interferon, and others.2, 3 In spite of these treatments, two small case series (n = 10, n = 17) have reported CEL-NOS to carry a poor prognosis with median survival of less than 2 years and high rates of leukemic transformation.2, 3
There is a paucity of population-based data in CEL-NOS.4 To date, no study has used the National Cancer Database (NCDB) to report availability of treatments received, comorbidity burden, and other risk factors. Our study utilizes SEER and the NCDB to better characterize the incidence and survival trends of patients diagnosed with CEL-NOS from 2004 to 2015.
SEER is a program of the National Cancer Institute that publishes cancer incidence and survival data covering ~34.6% of the US population.5 The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB is a nationwide oncology outcomes database for >1500 cancer programs in the US and Puerto Rico that captures 70% of all newly diagnosed cases of cancer in the US.6 The NCDB Participant Use File and SEER 18 registries were used to identify patients with ICD-O-3 diagnosis code 9964/3 from 2004 to 2015.
Using SEER, data on incidence rates (IRs), overall survival (OS), and relative survival (RS) were obtained. Incidence was age-adjusted to the U.S. 2000 standard population. RS was defined as the ratio of the proportion of observed survivors in a cohort of CEL-NOS patients to the proportion of expected survivors in a comparable set of individuals that did not have CEL-NOS, adjusting for the general survival of the US population for race, sex, age, and time when the diagnosis was established. IRs (cases/1 000 000) and RS were calculated using the SEER*Stat software (version 8.3.6; NCI, Bethesda, MD, USA), while descriptive statistics such as median, interquartile ranges (IQR), and OS was calculated by extracting and analyzing SEER data in JMP 14 (SAS institute Inc., Cary, NC, USA).
In the NCDB, the Deyo adaptation (1992) of Charlson's comorbidity index was used to calculate comorbid disease burden. For medications, per the NCDBʼs methodology, hormone therapy included prednisone, immunotherapy included interferon-alpha, and hydroxyurea, cladribine, and imatinib were classified as chemotherapy. According to the NCDB, "other treatment" was treatment that could not be defined. OS was calculated using the Kaplan-Meier method, while hazard ratios (HR) with confidence intervals (CI) were calculated using Cox proportional hazards model. Only variables that were significant in univariate analysis were included in a multivariate analysis. Statistical analyses for NCDB were performed using SAS version 9.0.
In SEER, there were a total of 373 patients. All patients were documented to have bone marrow involvement. The median age at diagnosis was 56 years (IQR 42-68.5); 229 (61%) were males. 223 (60%) were non-Hispanic White (NHW), 57 (15%) were non-Hispanic Black (NHB), 51 (14%) were Hispanic, and 36 (10%) were non-Hispanic Asian Pacific Islander (NHAPI). The overall IR of CEL-NOS was 0.4 and did not change significantly between years 2004-2015. The IRs according to gender and racial groups were: male 0.5, female 0.3, NHW 0.3, NHB 0.5, Hispanic 0.3, and NHAPI 0.3. IRs were not impacted by race or sex.
31 (8%) patients had subsequent malignancies. Among patients (<1%) who had subsequent acute myeloid leukemia (AML), transformation occurred at 17 and 51 months following diagnosis of CEL-NOS. Among the patients (<1%) who subsequently developed acute lymphocytic leukemia (ALL), transformation occurred at 4 and 13 months following diagnosis of CEL-NOS. Vital status was known for all patients and there were 253 deaths (68%) at the last known follow up. With a median follow up of 7.6 years (IQR 6.8-8.2), the median OS was not reached (NR). Among those who died, the median time to death was 12 months (IQR 3-41). In the general U.S. population, the expected survival for 12, 24, 36, 48 and 60 months was 98.5%, 97.0%, 95.4%, 93.8%, and 92.2%, respectively. In contrast, the RS for patients with CEL-NOS at the same time points was 88.8%, 84.4%, 83.3%, 80.0%, and 78.7% (Supplementary Appendix S1).
There were a total of 588 CEL-NOS patients in NCDB. Two-hundred eighteen patients (45%) were diagnosed at an academic institution. The median age at diagnosis was 57 years (range 19-90); 337 (57%) were males and 220 (37.4%) were ≥65 years old. The median OS was 11.8 years (IQR 3.1-NR) and the median follow up time was 5.7 years (IQR 2.9-8.8). 397 (67%) had CDS of 0, 115 (20%) had CDS of 1, and 76 (13%) had CDS of >1. 274 (51%) had government insurance, while 264 (49%) had private insurance. OS was 86%, 70%, and 56% at 1-, 5-, and 10-year respectively. The year of diagnosis was not associated with improved OS (HR: 0.95 [95% CI: 0.91-1.0], P = .058). The OS of those diagnosed at an academic institution was 90%, 73%, and 57% at 1-, 5-, and 10-year respectively and 79%, 61%, and 48% at non-academic institutions respectively. Two-hundred ninety-seven (52%) patients received chemotherapy. The median time of chemotherapy treatment from the day of diagnosis was 14 days (range 0-1186). The OS of those who received chemotherapy was 88% at 1 year and 73% at 5 years compared to 84% and 68% at 1- and 5-year for those who did not (P = .217). 210 (36%) received hormone therapy. The OS of those who received hormone therapy was 90% at 1 year and 76% at 5 years compared to 84% and 66% at 1- and 5-year for those who did not (P = .111). 17 (3%) patients received immunotherapy. Immunotherapy was not associated with improved OS (P = .254). Factors independently predicting inferior OS were age ≥65 years at the time of diagnosis (P < .001) and being diagnosed or treated at a non-academic center (P = .02). The Supplementary Appendix S1 shows the univariate and multivariate analysis, while the Figure 1 shows the survival estimates using the Kaplan-Meier method.
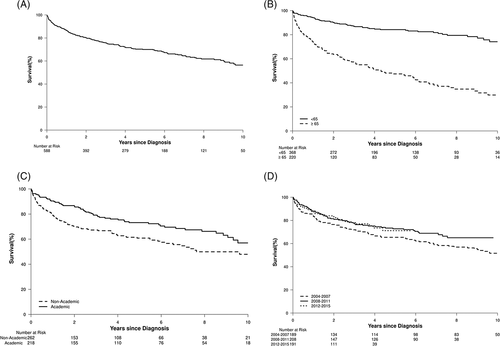
This is the first and largest retrospective study on CEL-NOS. In the existing literature, there are two case series (one done at our institution) that described a small cohort of patients, 10 and 17 respectively.2, 3 Comparing our population-based study to these two case series, we had a slightly younger median age of diagnosis (57 years vs 62-63 years) with a significant discrepancy between the median OS and rates of leukemic transformation. Two possible explanations can explain these differences.
The first is tertiary referral bias, wherein patients with more severe disease were evaluated at these two case series. In the two case series, comorbidities were not assessed while the majority of our patients had a CDS of 0. Both case series reported a higher rate of leukemic transformation, respectively 17% and 50%. In our study, the rate of transformation was low, but patients who did transform had poor survival. Finally, within our study, among patients who died, the median time from diagnosis to death was 12 months, which suggests there are a sub-group of patients who have much more severe disease compared to the rest.
The second explanation is that our cohort may inadvertently consist of patients with idiopathic hypereosinophilic syndrome or secondary eosinophilia. Establishing the diagnosis of CEL-NOS is challenging and resource-intensive. This is a significant limitation to population-based studies, as SEER and NCDB do not record testing for PDGFRA, PDGFRB, or FGFR1 rearrangements and PCM1-JAK2, ETV6-JAK2, or BCR-JAK2 fusion genes. Based on the OS data in the two small case series and assuming the diagnosis of CEL-NOS is more accurate at academic institutions, we would expect a significantly inferior OS at academic institutions compared to non-academic institutions. However, our results suggest otherwise.
Other limitations of SEER and NCDB are that they do not report individual granular data such as laboratory parameters and subsequent lines of treatment. Strengths of the study include the large cohorts in SEER and NCDB. The two cohorts are also in agreement from patient characteristic and OS perspective, which lends confidence to the accuracy of the data.
Our study confirms that CEL-NOS continues to be a rare leukemia and OS has not changed over the years. The discrepancy in overall survival between our national database study and existing case series suggests the need for future CEL-NOS studies to include information regarding diagnostic criterion, stratification of disease severity, and collaboration among academic institutions to allow for larger sample sizes to better characterize this disease.
CONFLICT OF INTERESTS
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
The authors declare no potential conflict of interest.




