Multiple myeloma: 2020 update on diagnosis, risk-stratification and management
Abstract
Disease overview
Multiple myeloma accounts for approximately 10% of hematologic malignancies.
Diagnosis
The diagnosis requires ≥10% clonal bone marrow plasma cells or a biopsy proven plasmacytoma plus evidence of one or more multiple myeloma defining events (MDE) namely CRAB (hypercalcemia, renal failure, anemia, or lytic bone lesions) features felt related to the plasma cell disorder, bone marrow clonal plasmacytosis ≥60%, serum involved/uninvolved free light chain (FLC) ratio ≥100 (provided involved FLC is ≥100 mg/L), or >1 focal lesion on magnetic resonance imaging (MRI).
Risk stratification
The presence of del(17p), t(4;14), t(14;16), t(14;20), gain 1q, or p53 mutation is considered high-risk multiple myeloma. Presence of any two high risk factors is considered double-hit myeloma; three or more high risk factors is triple-hit myeloma.
Risk-adapted initial therapy
In transplant eligible patients, induction therapy consists of bortezomib, lenalidomide, dexamethasone (VRd) given for approximately 3-4 cycles followed by autologous stem cell transplantation (ASCT). In high-risk patients, daratumumab, bortezomib, lenalidomide, dexamethasone (Dara-VRd) is an alternative to VRd. Selected standard risk patients can get additional cycles of induction, and delay transplant until first relapse. Patients not candidates for transplant are typically treated with VRd for approximately 8-12 cycles followed by lenalidomide; alternatively these patients can be treated with daratumumab, lenalidomide, dexamethasone (DRd).
Maintenance therapy
After ASCT, standard risk patients need lenalidomide maintenance, while bortezomib-based maintenance is needed for patients with high-risk myeloma.
Management of refractory disease
Most patients require a triplet regimen at relapse, with the choice of regimen varying with each successive relapse.
1 DISEASE OVERVIEW
Multiple myeloma accounts for 1% of all cancers and approximately 10% of all hematologic malignancies.1 Each year over 32 000 new cases are diagnosed in the United States, and almost 13 000 patients die of the disease.2 The annual age-adjusted incidence in the United States has remained stable for decades at approximately four per 100 000.3 Multiple myeloma is slightly more common in men than in women, and is twice as common in African-Americans compared with Caucasians.4 The median age of patients at the time of diagnosis is about 65 years.5
Unlike other malignancies that metastasize to bone, the osteolytic bone lesions in multiple myeloma exhibit no new bone formation.6 Bone disease is the main cause of morbidity and can be best detected using low-dose whole body computed tomography (WB-CT), fluoro-deoxyglucose (FDG) positron emission tomography/computed tomographic scans (PET/CT), or magnetic resonance imaging (MRI).7 Other major clinical manifestations are anemia, hypercalcemia, renal failure, and an increased risk of infections. Approximately 1% to 2% of patients have extramedullary disease (EMD) at the time of initial diagnosis, while 8% develop EMD later on in the disease course.8
Almost all patients with multiple myeloma evolve from an asymptomatic pre-malignant stage termed monoclonal gammopathy of undetermined significance (MGUS).9, 10 It is present in over 3% of the population above the age of 50,11, 12 and the prevalence is approximately 2-fold higher in blacks compared with whites.13, 14 And, MGUS progresses to multiple myeloma or related malignancy at a rate of 1% per year.15, 16 Note, MGUS is asymptomatic, and over 50% of individuals who are diagnosed with MGUS have had the condition for over 10 years prior to the clinical diagnosis.17 In some patients, an intermediate asymptomatic, but more advanced pre-malignant stage referred to as smoldering multiple myeloma, (SMM) can be recognized clinically.18 Smoldering multiple myeloma progresses to multiple myeloma at a rate of approximately 10% per year over the first 5 years following diagnosis, 3% per year over the next 5 years, and 1.5% per year thereafter. This rate of progression is influenced by the underlying cytogenetic type of disease; patients with t(4;14) translocation, del(17p), and gain(1q) are at a higher risk of progression from MGUS or SMM to multiple myeloma.19-21
2 DIAGNOSIS
The revised International Myeloma Working Group criteria for the diagnosis of multiple myeloma and related disorders are shown on Table 1.1 The diagnosis of multiple myeloma requires the presence of one or more myeloma defining events (MDE) in addition to evidence of either 10% or more clonal plasma cells on bone marrow examination or a biopsy-proven plasmacytoma. MDE consist of established CRAB (hypercalcemia, renal failure, anemia, or lytic bone lesions) features as well as three specific biomarkers: clonal bone marrow plasma cells ≥60%, serum free light chain (FLC) ratio ≥100 (provided involved FLC level is ≥100 mg/L), and more than one focal lesion on MRI. Each of the new biomarkers is associated with an approximately 80% risk of progression to symptomatic end-organ damage in two or more independent studies. The updated criteria represent a paradigm shift since they allow early diagnosis and initiation of therapy before end-organ damage.
| Disorder | Disease definition |
|---|---|
| Non-IgM monoclonal gammopathy of undetermined significance (MGUS) | All three criteria must be met:
|
| Smoldering multiple myeloma | Both criteria must be met:
|
| Multiple myeloma | Both criteria must be met:
|
| IgM monoclonal gammopathy of undetermined significance (IgM MGUS) | All three criteria must be met:
|
| Light chain MGUS | All criteria must be met:
|
| Solitary plasmacytoma | All four criteria must be met
|
| Solitary plasmacytoma with minimal marrow involvement b | All four criteria must be met
|
- a A bone marrow can be deferred in patients with low risk MGUS (IgG type, M protein <15 g/L, normal free light chain [FLC] ratio) in whom there are no clinical features concerning for myeloma.
- b Solitary plasmacytoma with 10% or more clonal plasma cells is considered as multiple myeloma.
- Source: Reproduced from Rajkumar et al.1
When multiple myeloma is suspected clinically, patients should be tested for the presence of M proteins using a combination of tests that should include a serum protein electrophoresis (SPEP), serum immunofixation (SIFE), and the serum FLC assay.22 Approximately 2% of patients with multiple myeloma have true non-secretory disease and have no evidence of an M protein on any of the above studies.5, 23 Bone marrow studies at the time of initial diagnosis should include fluorescent in situ hybridization (FISH) probes designed to detect t(11;14), t(4;14), t(14;16), t(6;14), t(14;20), trisomies, and del(17p) (see risk-stratification below).24 Conventional karyotyping to detect hypodiploidy and deletion 13 has value, but if FISH studies are done, additional value in initial risk-stratification is limited. Gene expression profiling (GEP) if available can provide additional prognostic value.25 Serum CrossLaps to measure carboxy-terminal collagen crosslinks (CTX) may be useful in assessing bone turnover and to determine adequacy of bisphosphonate therapy.26, 27 The extent of bone disease is best assessed by low-dose WB-CT or PET/CT imaging.7, 28 MRI scans are useful in patients with suspected SMM to rule out focal bone marrow lesions that can be seen before true osteolytic disease occurs. Also, MRI imaging is useful in assessing extramedullary disease, suspected cord compression, or when detailed imaging of a specific symptomatic area is needed. Conventional skeletal survey is less sensitive than low-dose WB-CT and PET/CT and recommended only if resources for more advanced imaging are not available.
The M protein is considered to be measurable if it is ≥1 g/dL in the serum and or ≥ 200 mg/day in the urine. The M protein level is monitored by SPEP and serum FLC assay to assess treatment response every month while on therapy, and every 3-4 months when off-therapy. The serum FLC assay is particularly useful in patients who lack a measurable M protein, provided the FLC ratio is abnormal and the involved FLC level is ≥100 mg/L.29 Urine protein electrophoresis is recommended at least once every 3-6 months, to follow the urine M protein level as well as to detect other renal complications that may result in albuminuria. Response to therapy assessment and minimal residual disease (MRD) evaluation is based on the revised International Myeloma Working Group uniform response criteria.30
3 MOLECULAR CLASSIFICATION
Although multiple myeloma is still considered a single disease, it is in reality a collection of several different cytogenetically distinct plasma cell malignancies (Table 2).31, 32 On FISH studies of the bone marrow, approximately 40% of multiple myeloma is characterized by the presence of trisomies in the neoplastic plasma cells (trisomic multiple myeloma), while most of the rest have a translocation involving the immunoglobulin heavy chain (IgH) locus on chromosome 14q32 (IgH translocated multiple myeloma).33-36 A small proportion of patients have both trisomies and IgH translocations. Trisomies and IgH translocations are considered primary cytogenetic abnormalities and occur at the time of establishment of MGUS. In addition, other cytogenetic changes termed secondary cytogenetic abnormalities arise along the disease course of multiple myeloma, including gain(1q), del(1p), del(17p), del (13), RAS mutations, and secondary translocations involving MYC. Both primary and secondary cytogenetic abnormalities can influence disease course, response to therapy, and prognosis. Importantly, the interpretation and impact of cytogenetic abnormalities in multiple myeloma vary depending on the disease phase in which they are detected (Table 3).37
| Subtype | Gene(s)/chromosomes affected a | Percentage of myeloma patients |
|---|---|---|
| Trisomic multiple myeloma | Recurrent trisomies involving odd-numbered chromosomes with the exception of chromosomes 1, 13, and 21 | 42 |
| IgH translocated multiple myeloma | 30 | |
| t(11;14) (q13;q32) | CCND1 (cyclin D1) | 15 |
| t(4;14) (p16;q32) | FGFR-3 and MMSET | 6 |
| t(14;16) (q32;q23) | C-MAF | 4 |
| t(14;20) (q32;q11) | MAFB | <1 |
| Other IgH translocations a | CCND3 (cyclin D3) in t(6;14) multiple myeloma | 5 |
| Combined IgH translocated/trisomic multiple myeloma | Presence of trisomies and any one of the recurrent IgH translocations in the same patient | 15 |
| Isolated Monosomy 14 | Few cases may represent 14q32 translocations involving unknown partner chromosomes | 4.5 |
| Other cytogenetic abnormalities in absence of IgH translocations or trisomy or monosomy 14 | 5.5 | |
| Normal | 3 |
- a Includes the t(6;14)(p21;q32) translocation, and rarely, other IgH translocations involving uncommon partner chromosomes.
- Source: Modified from Kumar et al.47
| Cytogenetic abnormality | Clinical setting in which abnormality is detected | |
|---|---|---|
| Smoldering multiple myeloma | Multiple myeloma | |
| Trisomies | Intermediate-risk of progression, median TTP of 3 y | Good prognosis, standard-risk MM, median OS 7-10 y Most have myeloma bone disease at diagnosis Excellent response to lenalidomide-based therapy |
| t(11;14) (q13;q32) | Standard-risk of progression, median TTP of 5 y | Good prognosis, standard-risk MM, median OS 7-10 y |
| t(6;14) (p21;q32) | Standard-risk of progression, median TTP of 5 y | Good prognosis, standard-risk MM, median OS 7-10 y |
| t(4;14) (p16;q32) | High-risk of progression, median TTP of 2 y | High-risk MM, median OS 5 y Needs early ASCT (if eligible), followed by bortezomib-based consolidation/maintenance |
| t(14;16) (q32;q23) | Standard-risk of progression, median TTP of 5 y | High-risk MM, median OS 5 y Associated with high levels of FLC and 25% present with acute renal failure as initial MDE |
| t(14;20) (q32;q11) | Standard-risk of progression, median TTP of 5 y | High-risk MM, median OS 5 y; Needs early ASCT (if eligible), followed by bortezomib-based consolidation/maintenance |
| Gain(1q21) | High-risk of progression, median TTP of 2 y | High-risk MM, median OS 5 y; Needs early ASCT (if eligible), followed by bortezomib-based consolidation/maintenance |
| Del(17p) | High-risk of progression, median TTP of 2 y | High-risk MM, median OS 5 y; Needs early ASCT (if eligible), followed by bortezomib-based consolidation/maintenance |
| Trisomies plus any one of the IgH translocations | Standard-risk of progression, median TTP of 5 y | May ameliorate adverse prognosis conferred by high risk IgH translocations, and del 17p |
| Isolated monosomy 13, or isolated monosomy 14 | Standard-risk of progression, median TTP of 5 y | Effect on prognosis is not clear |
| Normal | Low-risk of progression, median TTP of 7–10 y | Good prognosis, probably reflecting low tumor burden, median OS >7-10 y |
- Abbreviations: ASCT, autologous stem cell transplantation; FISH, fluorescent in situ hybridization, MM, multiple myeloma; OS, overall survival; SMM, Smoldering multiple myeloma; TTP, time to progression.
- Source: Modified from Rajan and Rajkumar.37
4 PROGNOSIS AND RISK STRATIFICATION
Survival estimates in multiple myeloma vary based on the source of the data. Data from randomized controlled trials using modern therapy show that the median survival in multiple myeloma is approximately 6 years.38 In the subset of patients eligible for ASCT, 4-year survival rates are more than 80%39; the median overall survival (OS) among these patients is approximately 8 years.40 Among elderly patients (age >75 years), median OS is lower, and is approximately 5 years.38 These numbers likely underestimate current survival probabilities since they predate the arrival of monoclonal antibodies and several other new agents that have been introduced in the last 3 to 5 years. On the other hand, they may be overestimates of the true population-based survival since they are derived from randomized controlled trials where patients with poor performance status and comorbidities are typically excluded. Nevertheless, these estimates are valuable benchmarks, and appear generalizable to newly diagnosed myeloma patients in good performance status.41
More precise estimation of prognosis requires an assessment of multiple factors. As in other cancers, OS in multiple myeloma is affected by host characteristics, tumor burden (stage), biology (cytogenetic abnormalities), and response to therapy.42, 43 Tumor burden in multiple myeloma has traditionally been assessed using the Durie-Salmon Staging (DSS)44 and the International Staging System (ISS).45, 46 Disease biology best reflected based on the molecular subtype of multiple myeloma (Table 2), the presence or absence of secondary cytogenetic abnormalities such as del(17p), gain(1q), or del(1p).24, 47 In addition to cytogenetic risk factors, two other markers that are associated with aggressive disease biology are elevated serum lactate dehydrogenase, and evidence of circulating plasma cells on routine peripheral smear examination (plasma cell leukemia). The Revised International Staging System (RISS) combines elements of tumor burden (ISS) and disease biology (presence of high risk cytogenetic abnormalities or elevated lactate dehydrogenase level), to create a unified prognostic index that and helps in clinical care as well as in comparison of clinical trial data (Table 4).48 In order to ensure uniform availability, only three widely available cytogenetic markers are used in the RISS; the Mayo Clinic mSMART risk stratification (www.msmart.org) (Table 5) has additional detail that is valuable in formulating a therapeutic strategy.
| Stage |
|---|
Stage 1 All of the following:
|
Stage II
|
Stage III Both of the following:
|
- Source: Derived from: Palumbo et al.48
| Risk group | Percentage of newly diagnosed patients with the abnormality |
|---|---|
| Standard risk | 75% |
| Trisomies | |
| t(11;14) | |
| t(6;14) | |
| High risk | 25% |
| t(4;14) | |
| t(14:16) | |
| t(14;20) | |
| del(17p) | |
| gain(1q) | |
| Double-hit: any two high-risk factors | |
| Triple-hit: any three or more high-risk factors |
Treated appropriately, the survival of patients with certain high risk categories can approach that of patients with standard risk disease. In a large trial using bortezomib-based induction, early ASCT, and bortezomib maintenance, the median OS of patients with del(17p) was approximately 8 years (8-year survival rate of 52%), and was identical to patients with standard risk multiple myeloma. In contrast, survival was lower for patients with t(4;14) translocation (8-year survival rate, 33%) and for patients with gain (1q) abnormality (8-year survival rate, 36%).40 These findings underscore the limitations of current risk stratification models in the context of modern therapy and highlight the need to stratify multiple myeloma based on individual cytogenetic groups rather than arbitrary heterogeneous risk categories.31
5 TREATMENT OF NEWLY DIAGNOSED MYELOMA
Survival in multiple myeloma has improved significantly in the last 15 years.49 The initial impact came from the introduction of thalidomide,50 bortezomib,51 and lenalidomide.52, 53 In the last decade, carfilzomib, pomalidomide, panobinostat, ixazomib, elotuzumab, daratumumab, isatuximab, and selinexor have been approved by the Food and Drug Administration (FDA) for the treatment of relapsed multiple myeloma, and promise to improve outcomes further. Numerous combinations have been developed using drugs that have shown activity in multiple myeloma, and the most commonly used regimens are listed in Table 6.54-76 These drugs work through a variety of mechanisms, some of which are not fully understood. Thalidomide, lenalidomide, and pomalidomide are termed immunomodulatory agents (IMiDs);they bind to cereblon and activate cereblon E3 ligase activity. This results in the rapid ubiquitination and degradation of two specific B cell transcription factors, Ikaros family zinc finger proteins Ikaros (IKZF 1) and Aiolos (IKZF3).77-79 They may cause direct cytotoxicity by inducing free radical mediated DNA damage.80 They also have anti-angiogenic, immunomodulatory, and tumor necrosis factor alpha inhibitory properties. Bortezomib, carfilzomib, and ixazomib are proteasome inhibitors.81-83 Elotuzumab targets SLAMF7; daratumumab and isatuximab target CD38 respectively.71, 84-86 Panobinostat is a deacetylase inhibitor.73, 87
| Regimen | Usual Dosing Schedule a |
|---|---|
| Thalidomide-dexamethasone (Td) b 54, 55 | Thalidomide 200 mg oral days 1-28 Dexamethasone 40 mg oral days 1, 8, 15, 22 Repeated every 4 wk |
| Lenalidomide-dexamethasone (Rd)56 | Lenalidomide 25 mg oral days 1-21 every 28 days Dexamethasone 40 mg oral days 1, 8, 15, 22 every 28 days Repeated every 4 wk |
| Pomalidomide-dexamethasone (Pom/Dex)57 | Pomalidomide 4 mg days 1-21 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 wk |
| Bortezomib-melphalan-prednisone (VMP) b 58-60 | Bortezomib 1.3 mg/m2 subcutaneous days 1, 8, 15, 22 Melphalan 9 mg/m2 oral days 1-4 Prednisone 60 mg/m2 oral days 1 to 4 Repeated every 35 days |
| Bortezomib-thalidomide-dexamethasone (VTd) b 61 | Bortezomib 1.3 mg/m2 subcutaneous days 1, 8, 15, 22 Thalidomide 100-200 mg oral days 1-21 Dexamethasone 20 mg oral on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 wk × 4 cycles as pre-transplant induction therapy |
| Bortezomib- cyclophosphamide-dexamethasone b(VCd or CyBord)62, 63 | Cyclophosphamide 300 mg/m2 orally on days 1, 8, 15 and 22 Bortezomib 1.3 mg/m2 subcutaneous on days 1, 8, 15, 22 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 wk c |
| Bortezomib-lenalidomide-dexamethasone (VRd) b 63, 64 | Bortezomib 1.3 mg/m2 subcutaneous days 1, 8, 15 Lenalidomide 25 mg oral days 1-14 Dexamethasone 20 mg oral on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 3 wk d |
| Carfilzomib-cyclophosphamide-dexamethasone (KCd) e 65 | Carfilzomib 20 mg/m2 (days 1 and 2 of cycle 1) and 27 mg/m2 (subsequent doses) intravenously on days 1, 2, 8, 9, 15, 16 Cyclophosphamide 300 mg/m2 orally on days 1, 8, 15 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 wk |
| Carfilzomib-lenalidomide-dexamethasone (KRd) e 66 | Carfilzomib 20 mg/m2 (days 1 and 2 of cycle 1) and 27 mg/ m2 (subsequent doses) intravenously on days 1, 2, 8, 9, 15, 16 Lenalidomide 25 mg oral days 1-21 Dexamethasone 40 mg oral days 1, 8, 15, 22 Repeated every 4 wk |
| Carfilzomib-pomalidomide-dexamethasone (KPd) e 67 | Carfilzomib 20 mg/m2 (days 1 and 2 of cycle 1) and 27 mg/m2 (subsequent cycles) intravenously on days 1, 2, 8, 9, 15, 16 Pomalidomide 4 mg oral on days 1-21 Dexamethasone 40 mg oral on days on days 1, 8, 15, 22 Repeated every 4 wk |
| Daratumumab-lenalidomide-dexamethasone (DRd)68 | Daratumumab 16 mg/kg intravenously weekly × 8 wk, and then every 2 wk for 4 months, and then once monthly Lenalidomide 25 mg oral days 1-21 Dexamethasone 40 mg intravenous days 1, 8, 15, 22 (given oral on days when no daratumumab is being administered) Lenalidomide-Dexamethasone repeated in usual schedule every 4 wk |
| Daratumumab-bortezomib-dexamethasone (DVd) b 69 | Daratumumab 16 mg/kg intravenously weekly × 8 wk, and then every 2 wk for 4 months, and then once monthly Bortezomib 1.3 mg/m2 subcutaneous on days 1, 8, 15, 22 Dexamethasone 40 mg intravenous days 1, 8, 15, 22 (given oral on days when no daratumumab is being administered) Bortezomib-Dexamethasone repeated in usual schedule every 4 wk |
| Daratumumab-pomalidomide-dexamethasone (DPd)70 | Daratumumab 16 mg/kg intravenously weekly × 8 wk, and then every 2 wk for 4 months, and then once monthly Pomalidomide 4 mg oral on days 1-21 Dexamethasone 40 mg intravenous days 1, 8, 15, 22 (given oral on days when no daratumumab is being administered) Repeated every 4 wk |
| Elotuzumab-lenalidomide-dexamethasone (ERd)71 | 10 mg/kg intravenously weekly × 8 wk, and then every 2 wk Lenalidomide 25 mg oral days 1-21 Dexamethasone per prescribing information Lenalidomide-Dexamethasone repeated in usual schedule every 4 wk |
| Ixazomib-lenalidomide-dexamethasone (IRd)72 | Ixazomib 4 mg oral days 1, 8, 15 Lenalidomide 25 mg oral days 1-21 Dexamethasone 40 mg oral days 1, 8, 15, 22 Repeated every 4 wk |
| Panobinostat-bortezomib b 73 | Panobinostat 20 mg oral three times a week × 2 wk Bortezomib 1.3 mg/m2 subcutaneous days 1, 8, 15 Repeated every 3 wk |
| Elotuzumab-pomalidomide-dexamethasone (EPd)74 | 10 mg/kg intravenously weekly × 8 wk, and then 20 mg/kg every 4 wk Pomalidomide 4 mg oral days 1-21 Dexamethasone per prescribing information Lenalidomide-Dexamethasone repeated in usual schedule every 4 wk |
| Isatuximab-pomalidomide-dexamethasone (Isa-Pd)75 | 10 mg/kg intravenously weekly × 4 wk, and then every 2 wk Pomalidomide 4 mg oral days 1-21 Dexamethasone per prescribing information Pomalidomide-Dexamethasone repeated in usual schedule every 4 wk |
| Selinexor-dexamethasone b 76 | Selinexor 100 mg/kg oral once weekly Dexamethasone 20 mg oral twice weekly |
- a All doses need to be adjusted for performance status, renal function, blood counts, and other toxicities.
- b Doses of dexamethasone and/or bortezomib reduced based on other data showing lower toxicity and similar efficacy with reduced doses; dose of selinexor reduced based on better tolerability with once weekly dosing in subsequent randomized trial; subcutaneous route of administration of bortezomib preferred based on data showing lower toxicity and similar efficacy compared to intravenous administration.
- c The day 22 dose of all three drugs is omitted if counts are low, or after initial response to improve tolerability, or when the regimen is used as maintenance therapy. When used as maintenance therapy for high risk patients, further delays can be instituted between cycles.
- d Omit day 15 dose if counts are low or when the regimen is used as maintenance therapy. When used as maintenance therapy for high risk patients, lenalidomide dose may be decreased to 10-15 mg per day, and delays can be instituted between cycles as done in total therapy protocols.
- e Carfilzomib can also considered in a once a week schedule of 56 mg/m2 on days 1, 8 and 15 every 28 days (cycle 1, day 1 should be 20 mg/ m2); day 8, 9 doses of carfilzomib can be omitted in maintenance phase of therapy after a good response to improve tolerability; KCd dosing lowered from that used in the initial trial which was conducted in newly diagnosed patients.
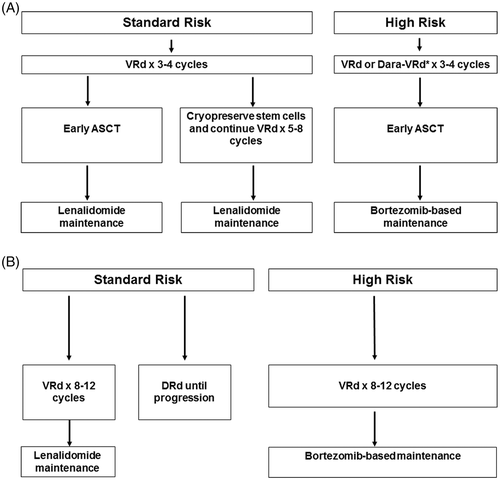
The approach to treatment of symptomatic newly diagnosed multiple myeloma is outlined in Figure 1 and is dictated by eligibility for ASCT and risk-stratification. The data to support their use from recent randomized trials using new active agents for multiple myeloma are provided in Table 7.38, 39, 88, 89 In order to initiate therapy, patients must meet criteria for multiple myeloma as outlined in Table 1. Early therapy with lenalidomide and dexamethasone or single-agent lenalidomide is beneficial in patients with high risk smoldering multiple myeloma, and is discussed separately.90, 91 There is an ongoing “cure versus control” debate on whether we should treat multiple myeloma with an aggressive multi-drug strategy targeting complete response (CR), or a sequential disease control approach that emphasizes quality of life as well as OS.92, 93
| Trial | Regimen | Number of patients | Overall response rate (%) | CR plus VGPR (%) | Progression-free survival (median in months) | P value for progression free survival | Overall survival (median in months) a | P value for overall survival |
|---|---|---|---|---|---|---|---|---|
| Durie et al38 | Rd | 229 | 72 | 32 | 31 | .002 | 64 | .025 |
| VRd | 242 | 82 | 43 | 43 | 75 | |||
| Attal et al39 | VRd | 350 | 97 | 77 | 36 | NR; 82% at 4 y | .87 | |
| VRd-ASCT | 350 | 98 | 88 | 50 | <.001 | NR; 81% at 4 y | ||
| Facon et al88 | Rd | 369 | 81 | 53 | 32 | NR | N/A | |
| DRd | 368 | 93 | 79 | NR; 71% at 30 mo | <.001 | NR | ||
| Moreau et al89 | VTd | 542 | 90 | 78 | NR; 85% at 18 mo | <.001 | NR; 90% at 30 mo | <.05 |
| Dara-VTd | 543 | 90 | 83 | NR; 93% at 18 mo | NR; 96% at 30 mo |
- Abbreviations: ASCT, autologous stem cell transplantation; CR, complete response; Dara-VTd, daratumumab, bortezomib, thalidomide, dexamethasone; DRd, daratumumab, lenalidomide, dexamethasone; N/A, not available; Rd, lenalidomide plus dexamethasone; VGPR, very good partial response; VRd, bortezomib, lenalidomide plus dexamethasone;VTd, bortezomib, thalidomide, dexamethasone.
- a Estimated from survival curves when not reported.
Recent data show that MRD negative status (as estimated by next generation molecular methods or flow cytometry) has favorable prognostic value.30 However, additional trials are needed to determine if changes in treatment need to be made based on MRD status. At present, MRD results are recommended mainly as a prognostic metric and not for used in making treatment decisions. We also need additional data to determine if MRD negativity can be used as a surrogate endpoint for regulatory approval, and if sustained MRD negativity may be a marker of cure in at least a subset of patients.32
5.1 Initial treatment in patients eligible for ASCT
Typically, patients are treated with approximately 3-4 cycles of induction therapy prior to stem cell harvest. After harvest, patients can either undergo frontline ASCT or resume induction therapy delaying ASCT until first relapse. There are many options for initial therapy, and the most common treatment regimens are discussed below. These regimens can also be used at the time of relapse. In general, the low-dose dexamethasone regimen (40 mg once a week) is preferred in all regimens to minimize toxicity. In a randomized trial conducted by the Eastern Cooperative Oncology Group (ECOG), the low-dose dexamethasone approach was associated with superior OS and significantly lower toxicity.56
5.1.1 Triplet regimens
Bortezomib, lenalidomide, dexamethasone (VRd) is the current standard of care for newly diagnosed multiple myeloma. In a randomized trial conducted by the Southwest Oncology Group (SWOG), response rates, PFS, and OS were significantly superior with VRd compared with Rd (Table 7).38 Stem cell collection with granulocyte stimulating factor (G-CSF) alone may be impaired when lenalidomide is used as induction therapy.94 Patients who have received more than 4-6 cycles of lenalidomide may need plerixafor for stem cell mobilization. All patients treated with lenalidomide require anti-thrombosis prophylaxis. Aspirin is adequate for most patients, but in patients who are at higher risk of thrombosis, either low-molecular weight heparin or warfarin is needed.95-97 If lenalidomide is not available for use as initial therapy or in the presence of acute renal failure, other bortezomib-containing regimens such as bortezomib-thalidomide-dexamethasone (VTd) or bortezomib-cyclophosphamide-dexamethasone (VCd) can be used instead of VRd. A recent randomized trial found that VTd results in superior response rates compared with VCd, but impact on long-term outcomes is not known.98 Therefore both are reasonable alternatives to VRd. Daratumumab, lenalidomide, dexamethasone (DRd) has shown significant activity in patients who are not candidates for transplantation, and is an additional alternative to VRd.88
In initial studies, peripheral neuropathy was a major concern with bortezomib therapy. Neuropathy with bortezomib can occur abruptly, and can be significantly painful and debilitating. However, the neurotoxicity of bortezomib can be greatly diminished by administering bortezomib once a week instead of twice-weekly,59, 60 and by administering the drug subcutaneously instead of the intravenous route.99 The once-weekly subcutaneous bortezomib schedule (Table 6) has made serious neuropathy an uncommon problem, and has made regimens such as VRd, VCd, and VTd much more tolerable. Bortezomib does not appear to have any adverse effect on stem cell mobilization.100
Two phase II trials reported results with carfilzomib when used in combination with lenalidomide and dexamethasone for newly diagnosed multiple myeloma.101, 102 However, there is concern for greater risk of serious toxicity with carfilzomib, and more data are needed. A randomized trial in the United States (referred to as the ENDURANCE trial) is currently ongoing comparing VRd vs KRd as initial therapy.
5.1.2 Quadruplet regimens
Quadruplet regimens containing daratumumab, a monoclonal antibody targeting CD38 are showing promise. In one randomized trial, daratumumab, bortezomib, thalidomide, dexamethasone (Dara-VTd) showed superior response rates, progression-free survival (PFS), and a trend to better OS compared with VTd.89 A randomized phase II trial found that the addition of daratumumab to VRd increases the rate and depth of response to therapy.103 In these trials, as expected, the benefit of daratumumab in terms of surrogate endpoints was more pronounced in the standard risk patients, a positive effect was nevertheless seen in both standard and high risk disease. Phase III data on the incremental PFS and OS benefit with quadruplet regimens over the current standard of VRd is awaited. Therefore, it is prudent to restrict the use of quadruplet regimens to transplant eligible patients with high risk double or triple hit myeloma, until we have clear OS data to justify adding potential long-term costs and risks to standard risk patients who currently have excellent outcomes with the VRd triplet. Trials with other quadruplet regimens are ongoing. A randomized trial to determine the patient subset that can benefit most from quadruplets is also expected to open soon in the United States.
5.1.3 Multi-drug combinations
Besides the regimens discussed above, other options include anthracycline-containing regimens such as bortezomib, doxorubicin, dexamethasone (PAD)40 or multi-agent combination chemotherapy regimens, such as VDT-PACE (bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide).104, 105 These regimens are particularly useful in patients with aggressive disease such as plasma cell leukemia or multiple extramedullary plasmacytomas. Several other regimens have been tested in newly diagnosed multiple myeloma, but there are no clear data from randomized controlled trials that they have an effect on long-term endpoints compared with the regimens discussed earlier.
5.1.4 Recommendations
- In standard-risk patients eligible for ASCT, I favor VRd as initial therapy for 3-4 cycles, followed by ASCT and lenalidomide maintenance therapy. In patients who are tolerating therapy and responding well, an alternative is VRd for 8 to 12 cycles followed by lenalidomide maintenance therapy. In such patients stem cells must be collected for cryopreservation after the first 3-4 cycles of VRd, and ASCT must be considered at first relapse.
- In high-risk patients, especially those with double-hit or triple-hit myeloma, I favor Dara-VRd as initial therapy for 3-4 cycles followed by ASCT and then bortezomib-based maintenance (eg, bortezomib every 2 weeks, or a low intensity VRd regimen).
- In patients presenting with acute renal failure suspected to be secondary to light-chain cast nephropathy, I prefer VCd or VTd as initial therapy in conjunction with plasma exchange (or dialysis with high-cut-off filter). Plasma exchange is continued daily until the serum FLC levels are less than 50 mg/dL and then repeated as needed till chemotherapy is fully effective.
- In patients presenting with plasma cell leukemia or multiple extramedullary plasmacytomas, I prefer VDT-PACE as initial therapy followed by ASCT and then maintenance with a bortezomib-based regimen.
- Once weekly subcutaneous bortezomib is preferred in most patients for initial therapy, unless there is felt to be an urgent need for rapid disease control.
- Dexamethasone 40 mg once a week (low-dose dexamethasone) is preferred in most patients for initial therapy, unless there is felt to be an urgent need for rapid disease control.
5.2 Initial treatment in patients not eligible for ASCT
In patients with newly diagnosed multiple myeloma who are not candidates for ASCT due to age or other comorbidities, the major options for initial therapy are VRd and DRd. Although melphalan-based regimens have been extensively tested in these patients, they are not recommended due to concerns about stem cell damage and secondary myelodysplastic syndrome and leukemia. In the United States transplant eligibility is not determined by a strict age cut-off, and many patients enrolled in the melphalan-based clinical trials would be considered candidates for ASCT.
5.2.1 Bortezomib-based regimens
Therapy with VRd has shown a survival benefit compared with Rd, and is the preferred choice for initial therapy in patients who are not candidates for ASCT (Table 7).38 So, VRd is administered for approximately 8-12 cycles, followed by maintenance therapy. In patients in whom initial therapy with VRd is not possible mainly for logistical reasons (such as problems with compliance due to need for parenteral administration), ixazomib can be considered in place of bortezomib. In frail elderly patients, a lower dose of lenalidomide should be used; dexamethasone may be started at 20 mg once a week, then reduced as much as possible after the first 4-6 cycles, and discontinued after the first year.
5.2.2 DRd
Note, DRd has been recently approved for patients with newly diagnosed myeloma, based on the results of an international multicenter randomized trial.88 PFS at 30 months was higher with DRd compared to Rd, 70.6% vs 55.6%, P < .001. MRD negative rates were also superior, 24.2% vs 7.3%, P < .001. DRd is an alternative to VRd in this setting. However, unlike VRd where the triplet regimen is only used for a limited duration, therapy with DRd requires treatment with all three drugs until progression which makes this a much more expensive regimen in the long-term.106
5.2.3 Alkylator-based regimens
Melphalan-based regimens are considered only if there are problems with access to lenalidomide. Even in these situations, the risks of melphalan can be reduced by using cyclophosphamide instead, and studies show this substitution does not alter efficacy.107 Thus, the VCd regimen can be considered as a minor modification of the VMP regimen, in which cyclophosphamide is used as the alkylating agent in place of melphalan. This variation has the advantage of not affecting stem cell mobilization, and dosing is more predictable. A randomized trial found superior PFS and OS with a four-drug regimen of Dara plus VMP compared with VMP in a randomized phase III trial, but the contribution of the fourth drug to the induction component cannot be ascertained from this trial.108
5.2.4 Recommendations
- In standard-risk patients, I prefer VRd as initial therapy administered for approximately 8-12 cycles, followed by lenalidomide maintenance. DRd is an alternative to VRd but adds cost and toxicity of long-term triplet therapy.
- In high-risk patients, I favor VRd as initial therapy for approximately 8-12 cycles followed by bortezomib-based maintenance (eg, bortezomib every 2 weeks, or a low intensity VRd regimen).
5.3 Hematopoietic stem cell transplantation
5.3.1 Autologous stem cell transplantation (ASCT)
ASCT improves median OS in multiple myeloma by approximately 12 months.109-112 However, randomized trials found similar OS with early ASCT (immediately following four cycles of induction therapy) vs delayed ASCT (at the time of relapse as salvage therapy).113-115 A trial by the Intergroupe Francophone du Myelome (IFM) compared early vs delayed ASCT in patients treated with VRd followed by lenalidomide maintenance.39 Patients were randomized to receive either VRd (three cycles) followed by ASCT and then VRd consolidation (two cycles) vs VRd × eight cycles with ASCT reserved for relapse. Both arms received lenalidomide maintenance for 1 year. A significant improvement in PFS was seen as expected with early ASCT, but this has so far not translated into a difference in OS (Table 7). Based on these results, it is reasonable to consider a delayed ASCT in patients with standard-risk multiple myeloma who prefer such an approach for personal and logistic reasons.
The role of tandem (double) ASCT is unclear. In earlier randomized trials, an improvement in OS was seen in two studies,116, 117 but other studies failed to show such an improvement.118, 119 More recent data are available from two other randomized trials are also inconclusive. In a trial conducted in Europe, an improvement in PFS and OS was seen with tandem ASCT in both standard and high risk patients.120 However, no survival benefit has been seen so far in a randomized trial, conducted in the United States by the Bone Marrow Transplantation Clinical Trials Network (BMT-CTN), in standard or high risk multiple myeloma (BMT-CTN 0702 trial).121 The US trial more likely reflects the impact of tandem ASCT in the context of modern therapy when most new options for salvage are available. Thus routine tandem ASCT is not recommended outside of a clinical trial setting.
5.3.2 Post-transplant consolidation
Consolidation therapy is a term used for the administration of a short course of therapy, usually with two or more drugs, prior to the start of long-term maintenance. The BMT-CTN 0702 trial had an arm that investigated the benefit of post-transplant consolidation therapy, followed by lenalidomide maintenance vs lenalidomide maintenance alone.121 In this trial, additional cycles of VRd chemotherapy administered as consolidation after ASCT did not result in significant benefit. Unlike earlier trials, the BMT-CTN 0702 trial specifically isolated the effect of consolidation and is therefore more compelling than trials where one could not ascertain the precise added value of consolidation therapy on PFS and OS. Consolidation therapy after ASCT is not recommended and patients should proceed to standard low-intensity maintenance therapy.
5.3.3 Allogeneic transplantation
The role of allogeneic and non-myeloablative-allogeneic transplantation in multiple myeloma is controversial with studies showing conflicting results.122, 123 The treatment related mortality (TRM) rate (10%-20%) and GVHD rates are fairly high.124 Although allogenic transplantation should still be considered as investigational, it may be a consideration for young patients with high-risk disease, who are willing to accept a high TRM and the unproven nature of this therapy for a chance at better long-term survival.
5.3.4 Recommendations
- ASCT should be considered in all eligible patients. But in standard-risk patients responding well to therapy, ASCT can be delayed until first relapse provided stem cells are harvested early in the disease course.
- Tandem ASCT is not recommended outside of clinical trials
- Allogeneic transplantation as frontline therapy should be considered investigational.
5.4 Maintenance therapy
Maintenance therapy is indicated following ASCT. Maintenance therapy should also be considered in following completion of 8-12 cycles of initial therapy in patients treated without ASCT. Lenalidomide is the standard of care for maintenance therapy for most patients.125-130 In a meta-analysis of randomized trials, a significant improvement in PFS and OS was seen with lenalidomide maintenance compared with placebo or no therapy.131 Lenalidomide maintenance is associated with a 2-3-fold increase in the risk of second cancers and patients must be counseled in this regard and monitored.
The impact of lenalidomide maintenance in patients with high risk multiple myeloma is unclear. In a meta-analysis, no significant OS benefit was seen in these subsets of high risk patients.131 However, in a more recent trial that was not part of the meta-analysis, benefit was seen in high risk patients.132 Bortezomib administered every other week has been shown to improve OS, particularly in patients with del(17p).128 Bortezomib-based maintenance is preferable for high-risk patients. This can either consist of bortezomib alone given every other week, or low intensity VRd, to capture the effect of bortezomib and lenalidomide.133 In patients unable to access or tolerate bortezomib, ixazomib is a reasonable alternative that has shown benefit in a placebo controlled randomized trial.134
Among patients who did not undergo upfront ASCT, based on the results of the SWOG trial, maintenance therapy with lenalidomide should be considered in patients who are in good performance status after completion of initial 8-12 cycles of triplet therapy.
Although the benefit of maintenance is now established, data on optimal duration are lacking. We also need to consider the cost, toxicity, and inconvenience of long-term indefinite maintenance therapy. Many patients seek a drug-free interval. An ECOG trial is comparing lenalidomide maintenance given until progression vs a limited duration of 2 years. Trials are also examining if the duration of maintenance can be modified based on MRD results.
5.4.1 Recommendations
- I recommend lenalidomide maintenance for standard-risk patients following ASCT. I also recommend lenalidomide maintenance following 8-12 cycles of VRd among patients who did not receive ASCT as part of initial therapy.
- I recommend maintenance with bortezomib alone or low intensity VRd for patients with high-risk multiple myeloma.
6 TREATMENT OF RELAPSED MULTIPLE MYELOMA
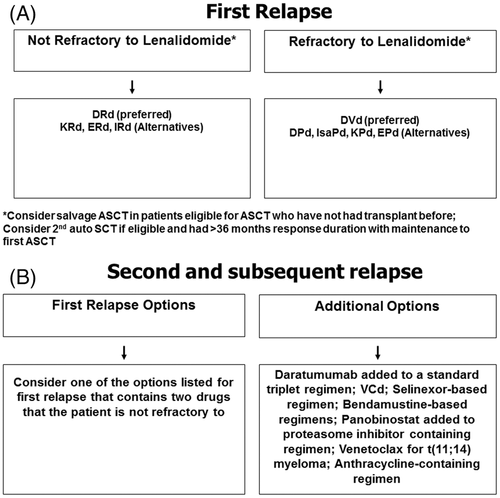
Almost all patients with multiple myeloma eventually relapse. The remission duration in relapsed multiple myeloma decreases with each regimen.135 The median PFS and OS in patients with relapsed multiple myeloma refractory to lenalidomide and bortezomib was low prior to the introduction of daratumumab respectively.136 The choice of a treatment regimen at relapse is complicated and is affected by many factors including the timing of the relapse, response to prior therapy, aggressiveness of the relapse, and performance status (TRAP). Patients are eligible for an ASCT should be considered for the procedure if they have never had one before, or if they have had an excellent remission duration with the first ASCT defined as a remission of at least 36 months or longer with maintenance. In terms of drug therapy, a triplet regimen containing at least two new drugs that the patient is not refractory to should be considered. An approach to the treatment of relapsed multiple myeloma is given in Figure 2. Major regimens used in the treatment of multiple myeloma, including relapsed disease are listed in Table 6. Recent advances in the treatment of relapsed multiple myeloma, including new active agents and results of major randomized trials are discussed below (Table 8).66, 68, 69, 71-73, 75, 137-140 One important consideration is that the lenalidomide-containing regimens listed in Table 8 were tested mainly in patient populations who were not previously exposed to lenalidomide. In contrast, current clinical practice typically consists of patients who have been treated with lenalidomide and are often relapsing while on a lenalidomide-containing regimen. In patients who are considered refractory to lenalidomide, one option is to consider pomalidomide-based regimens.
| Trial | Regimen | Number of patients | Overall response rate (%) | CR plus VGPR (%) | Progression-free survival (median in months) | P value for progression free survival | Overall survival a (median in months) | P value for overall survival |
|---|---|---|---|---|---|---|---|---|
| Stewart et al.66, 137 | Rd | 396 | 67 | 14 | 18 | 40 | .04 | |
| KRd | 396 | 87 | 32 | 26 | <.0001 | 48 | ||
| Dimopoulos et al.68 | Rd | 283 | 76 | 44 | 18.4 | <.001 | N/A; 87% at 1 y | NS |
| DRd | 286 | 93 | 76 | NR | N/A; 92% at 1 y | |||
| Palumbo et al.69 | Vd | 247 | 63 | 29 | 7.2 | <.001 | N/A; 70% at 1 y | .30 |
| DVd | 251 | 83 | 59 | NR | N/A; 80% at 1 y | |||
| Lonial et al.71, 141 | Rd | 325 | 66 | 28 | 15 | 40 | N/A | |
| Elo-Rd | 321 | 79 | 33 | 19 | <.001 | 44 | .03 | |
| Moreau et al.72 | Rd | 362 | 72 | 7 | 15 | N/A | N/A | |
| IRd | 360 | 78 | 12 | 21 | .012 | N/A | ||
| San Miguel et al.73, 142 | Vd | 381 | 55 | 6 | 8.1 | 36 | .54 | |
| Pano-Vd | 387 | 61 | 11 | 12 | <.0001 | 40 | ||
| Attal et al.75 | Pd | 153 | 35 | 9 | 6.5 | NR; 63% at 1 y | .06 | |
| Isa-Pd | 154 | 60 | 32 | 11.5 | <.001 | NR; 72% at 1 y | ||
| Dimopoulos et al.139, 140 | Vd | 465 | 63 | 6 | 9 | 40 | .01 | |
| Kd | 464 | 77 | 13 | 19 | <.0001 | 48 |
- Abbreviations: CR, complete response; Dex, high dose dexamethasone; DRd, daratumumab, lenalidomide, dexamethasone; DVd, daratumumab, bortezomib, dexamethasone; Elo-Rd, Elotuzumab, lenalidomide, dexamethasone; IRd, ixazomib, lenalidomide, dexamethasone; Isa-Pd, isatuximab, pomalidomide, dexamethasone; Kd, carfilzomib, dexamethasone; KRd, carfilzomib, lenalidomide, dexamethasone; N/A, not available; NS, not significant; Pano-Vd, panobinostat, bortezomib, dexamethasone; Pd, pomalidomide, dexamethasone; Rd, lenalidomide plus dexamethasone; Vd, bortezomib, dexamethasone; VGPR, very good partial response.
- a Estimated from updated publications when available; estimated from survival curves when not reported.
6.1 Bortezomib-based regimens
These regimens are appropriate for patients who received a bortezomib-based triplet for a period of time, and then stopped therapy. In these patients if relapse occurs after a reasonable period of remission off all therapy, then restarting the same (or similar) bortezomib-based triplet is reasonable and also carries lower cost and risk. As in newly diagnosed multiple myeloma, VRd, VCd, and VTd are active regimens in relapsed disease.143, 144
6.2 Daratumumab
Daratumumab targeting CD38 has shown promise in relapsed, refractory multiple myeloma.84 In a phase II trial, daratumumab as a single-agent was produced a response rate of approximately 30% in heavily pre-treated patients.85 Based on these findings, daratumumab was first granted accelerated approval by the FDA in 2015 for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent. Subsequently three other daratumumab-based combinations have shown efficacy and have been approved by the FDA for relapsed disease. These include DRd, daratumumab, bortezomib, dexamethasone (DVd), and daratumumab, pomalidomide, dexamethasone (DPd) (Table 8). The various triplets available for use in relapsed disease have not been compared head-to-head, daratumumab-based regimens appear to have the greatest reduction in risk of progression, and may be preferred for first relapse subject to availability and cost considerations.145
6.3 Carfilzomib
Carfilzomib is a novel keto-epoxide tetrapeptide proteasome inhibitor initially approved in 2013 for the treatment of relapsed refractory multiple myeloma in patients who have been previously treated with lenalidomide and bortezomib. The KRd regimen has been shown to be effective in a randomized trial, and is a major option for the treatment of relapsed disease (Table 8).66 In another randomized trial carfilzomib plus dexamethasone (Pd) was associated with an improvement in PFS and OS compared with bortezomib Pd in relapsed multiple myeloma.139, 140 However, the dose of carfilzomib used in this trial (56 mg/m2) is twice the standard dose, and carries a much higher cost compared with bortezomib. Carfilzomib is typically administered twice-weekly at a dose of 27 mg/m2 (refer to Table 6), but a once-weekly schedule of 56 mg/m2 may be equally effective and safe, and more convenient when used in triplet combinations.146 Carfilzomib carries a lower risk of neurotoxicity than bortezomib, but a small proportion (5%) of patients can experience serious cardiac side effects. Carfilzomib-based regimens are important options at relapse, and can work well even in patients who are refractory to a bortezomib-containing regimen.
6.4 Pomalidomide
Pomalidomide is an analog of lenalidomide and thalidomide initially approved in 2013 for the treatment of relapsed refractory multiple myeloma. It has significant activity in relapsed refractory multiple myeloma, even in patients failing lenalidomide.147, 148 Response rate with pomalidomide Pd in patients refractory to lenalidomide and bortezomib is approximately 30%.57, 149 In a randomized trial, Pd was found superior to high-dose dexamethasone in patients refractory to other forms of therapy for multiple myeloma (Table 8).138 Pomalidomide-containing triplet regimens such as DPd and carfilzomib, pomalidomide, dexamethasone (KPd) are active and are important options at relapse for patients who are considered lenalidomide-refractory.70, 150 In frail patients and in those with indolent relapse, the doublet regimen of Pd is a reasonable option.
6.5 Elotuzumab
Elotuzumab, a monoclonal antibody targeting the signaling lymphocytic activation molecule F7 (SLAMF7).71 Unlike daratumumab, elotuzumab does not have single-agent activity but shows synergistic activity when combined with Rd (Table 8).71 Elotuzumab is well tolerated, and was initially approved in 2015 by the FDA to be given in combination with Rd for the treatment of patients with multiple myeloma who have received one to three prior therapies. However, elotuzumab may be more active in combination with pomalidomide than with lenalidomide. In a randomized trial conducted in patients refractory to lenalidomide and a proteasome inhibitor elotuzumab, pomalidomide, dexamethasone (EPd) was superior to Pd; median PFS 10.3 vs 4.7 months, P = .008. Based on this trial, EPd has been approved by the FDA for patients with myeloma who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
6.6 Ixazomib
Ixazomib is an oral proteasome inhibitor that is active in both the relapsed refractory setting and in newly diagnosed multiple myeloma.72, 151 It has the advantage of once-weekly oral administration. Compared with bortezomib it has more gastrointestinal adverse events, but lower risk of neurotoxicity. In a randomized controlled trial in relapsed multiple myeloma, ixazomib, lenalidomide, dexamethasone (IRd) was found to improve PFS compared with Rd (Table 8).72 Based on these results ixazomib was initially approved by the FDA in 2015 to be given in combination with Rd for the treatment of patients with multiple myeloma who have received at least one prior therapy.
6.7 Selinexor
Selinexor blocks exportin 1 (XPO1), and leads to the accumulation and activation of various tumor suppressor proteins and the inhibition of nuclear factor kappaB. In one phase II trial, oral selinexor Pd was found to have a response rate of 26% in patients refractory to at least one proteasome inhibitor, one immunomodulatory agent, and daratumumab.76 Major side effects include thrombocytopenia, fatigue, nausea, and anorexia. In a recent randomized trial, Selinexor has been granted accelerated approval by the FDA for patients with relapsed refractory myeloma who have received at least four prior therapies, and whose disease is resistant to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
6.8 Isatuximab
Istatuximab is a monoclonal antibody targeting CD38 that has shown promise in relapsed, refractory multiple myeloma. In a randomized trial, isatuximab, pomalidomide, dexamethasone (Isa-Pd) was associated with better PFS compared to Pd in patients with relapsed and refractory multiple myeloma; median PFS 11.5 months vs 6.5 months, P = .001.75 Based on these data, isatuximab has been approved by the FDA for the treatment of relapsed refractory myeloma in patients who have received at least two previous lines of treatment, including lenalidomide and a proteasome inhibitor. Isatuximab provides an alternative to daratumumab in myeloma, and further studies will define its place in myeloma treatment.
6.9 Doxorubicin and liposomal doxorubicin
Anthracyclines have marginal single-agent activity in multiple myeloma. A phase III randomized trial found that median time to progression (TTP) was superior with bortezomib plus pegylated liposomal doxorubicin compared with bortezomib alone, 9.3 months vs 6.5 months, respectively, P < .001.152 The OS at 15 months was also superior, 76% compared with 65%, respectively, P = .03. Despite this study, liposomal doxorubicin is infrequently used in the treatment of relapsed multiple myeloma given availability of other active agents. Doxorubicin-containing regimens such as PAD or VDT-PACE may be useful in the treatment of patients with aggressive multiple myeloma refractory to other standard myeloma agents.
6.10 Panobinostat
Panobinostat is a pan-deacetylase inhibitor initially approved by the FDA in 2015 for the treatment of patients with multiple myeloma who have received at least two prior standard therapies, including bortezomib and an immunomodulatory agent.73 Its putative mechanism of action is blockade of the aggresome pathway, an alternative route for cells to bypass the lethal effects of proteasome inhibition. By combining bortezomib and panobinostat, there is simultaneous blockade of both proteasome and aggresome pathways.153, 154 In a randomized trial of 768 patients, bortezomib/dexamethasone plus panobinostat was associated with superior PFS compared with bortezomib/dexamethasone plus placebo.73 However, panobinostat therapy is associated with grade 3 diarrhea in approximately 25% of patients, and care should be exercised when using this drug. I recommend a lower initial dose of panobinostat than the approved starting dose, and that bortezomib be used in the once-weekly subcutaneous schedule rather than the twice weekly regimen used in the pivotal trial (Table 6).
6.11 Other options
Despite the multiple options available, most patients eventually become refractory to all drug classes. Some additional options to consider for relapsed disease in refractory multiple myeloma include the use of an alkylator containing regimen such as VCd, or a quadruplet regimen in which a monoclonal antibody is added to a standard triplet regimen such as VRd or KRd. Other options include selinexor-containing regimens, bendamustine-containing regimens such as bendamustine, lenalidomide, dexamethasone or bendamustine, bortezomib, dexamethasone,155, 156 or the addition of panobinostat to a proteasome-inhibitor containing regimen. For young high-risk patients with a suitable donor, allogeneic transplantation is an option as well.
Venetoclax is not approved for use in multiple myeloma, but is commercially available, and appears to have single-agent activity in patients with t(11;14) subtype of multiple myeloma.157 However, the results of a recent randomized trial found significantly higher mortality with venetoclax in relapsed myeloma, despite producing deeper responses and better PFS.158 Therefore, venetoclax is best considered investigational, and its use should be restricted to patients with t(11;14) who have relapsed disease and limited options.
6.12 Emerging options
There are several investigational approaches that are promising and patients should be considered for clinical trials investigating these approaches. Two of the most exciting options include antigen receptor T cells (CAR-T) targeting B cell maturation antigen (BCMA) such as bb2121,159 and belantamab mafodotin (a humanized anti-BCMA antibody that is conjugated to monomethyl auristatin-F, a microtubule disrupting agent).160 Another option that is promising is the use of a bispecific T cell engager, such as AMG 701.
6.13 Recommendations
- Patients who are eligible for ASCT should consider ASCT as salvage therapy at first relapse if they have never had a transplant before, or if they have had a prolonged remission with the first ASCT.
- If relapse occurs more than 6 months after stopping therapy, the initial treatment regimen that successfully controlled the multiple myeloma initially can be re-instituted when possible.
- At first relapse, for patients who are not refractory to lenalidomide, my preferred option is DRd. Alternatives include KRd, IRd, and ERd.
- At first relapse, for patients who are refractory to lenalidomide, my preferred option is DVd. Alternatives include pomalidomide-based combinations such as DPd, Isa-Pd, KPd, or EPd.
- Patients who are frail with an indolent relapse can be treated with oral IRd.
- At second or higher relapse, I switch to a triplet regimen that contains at least two new drugs that the patient is not refractory to.
- Additional options to consider in patients with multiple relapses and disease that is refractory to conventional regimens include VCd, bendamustine-based regimens, the addition of panobinostat to a proteasome-inhibitor containing regimen, quadruplet daratumumab-containing regimens, multi-drug chemotherapy regimens, allogenic transplantation in young high risk patients with a suitable donor, and venetoclax in patients with t(11;14) multiple myeloma.
- Patients with more aggressive relapse with plasma cell leukemia or extramedullary plasmacytomas often require therapy with a multi-drug anthracycline containing regimen such as VDT-PACE.
- The duration of therapy has not been well addressed in relapsed multiple myeloma, and in some regimens such as those employing parenteral proteasome inhibitors it may be reasonable to stop therapy once a stable plateau has been reached, in order to limit minimize risks of serious toxicity.
7 SMOLDERING MULTIPLE MYELOMA
Smoldering multiple myeloma is a stage that is clinically positioned between MGUS and multiple myeloma.161 It comprises a heterogeneous group of patients, some of whom have multiple myeloma which has not yet manifested with MDEs, and some who have premalignant MGUS. Patients with SMM have a risk of progression of approximately 10% per year for the first 5 years, 3% per year for the next 5 years, and 1% per year thereafter.18 Patients with the highest risk of progression (ultra-high risk) have now been reclassified as having multiple myeloma by the new IMWG criteria.1 Within the current definition of SMM (Table 1), there are two groups of patients: high risk (25% per year risk of progression in the first 2 years) and low risk (~5% per year risk of progression).161 Risk factors for high risk SMM are given on Table 9.162 Presence of two or three of these factors is associated with a median TTP to multiple myeloma of approximately 2 years, and is considered high risk SMM (Mayo 2018 criteria).
| Mayo 2018 criteria |
| Any 2-3 of the following: |
| Serum M protein >2 g/dL |
| Serum FLC ratio (involved/uninvolved) >20 |
| Serum M protein ≥30 g/L |
| Bone marrow plasma cells >20% |
| Other high risk factors |
| Progressive increase in M protein level (evolving type of SMM) b |
| Bone marrow clonal plasma cells 50%-60% |
| t (4;14) or del 17p or 1q gain |
| Increased circulating plasma cells |
| MRI with diffuse abnormalities or with one focal lesion |
| PET-CT with focal lesion with increased uptake but without underlying osteolytic bone destruction |
- Abbreviations: M, monoclonal; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography; SMM, smoldering multiple myeloma.
- a Note that the term smoldering multiple myeloma excludes patients without end-organ damage who meet the revised definition of multiple myeloma, namely clonal bone marrow plasma cells ≥60% or serum free light chain (FLC) ratio ≥100 (plus measurable involved FLC level ≥100 mg/L), or more than one focal lesion on MRI. The risk factors listed in this table variables associated with a higher risk of progression of SMM, and identify patients who need close follow up and consideration for clinical trials. Patients who are high risk by Mayo 2018 criteria are candidates for prophylactic therapy with lenalidomide or lenalidomide plus dexamethasone in the absence of clinical trials.
- b Increase in serum monoclonal protein by ≥25% on two successive evaluations within a 6 months period.
Early studies in SMM failed to show an advantage to preventive intervention, but were limited by lack of power, safe and effective drugs, and a risk-adapted strategy.163, 164 A randomized trial conducted in Spain found that patients with high risk SMM had significant prolongation of PFS and OS with Rd compared with observation.90, 165 A recent ECOG randomized trial provided additional confirmation, and found that early therapy with lenalidomide prolonged time to end-organ damage in patients with high risk SMM.91 Based on these two trials, patients with newly diagnosed high risk SMM patients should be considered for early intervention with lenalidomide or lenalidomide Pd. An ongoing ECOG randomized trial is testing whether a standard myeloma therapeutic triplet (DRd) will be superior to prophylactic doublet therapy with lenalidomide Pd. They are also candidates for clinical trials testing early intervention, some of which are testing intensive therapy with curative intent.166
7.1 Recommendations
- I recommend lenalidomide or lenalidomide plus dexamethasone for patients with newly diagnosed high risk SMM. All other patients should be observed without therapy.
ACKNOWLEDGMENTS
Supported in part by grants CA 107476, CA 168762, and CA186781 from the National Cancer Institute, Rockville, MD, USA.
CONFLICT OF INTEREST
SVR declares no conflict of interest.
AUTHOR CONTRIBUTIONS
S.V.R. conceived of the paper, researched the literature, and wrote the manuscript.




